Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
The predictive value of ‘red flags’ as milestones of psychomotor development of premature babies – preliminary study
1
Department of Rehabilitation, Physiotherapy and Balneotherapy, Faculty of Health Sciences, Medical University, Lublin, Poland
2
Department of Pediatric Rehabilitation, University Children’s Hospital, Lublin, Poland
3
Department of Children Orthopedics, Medical University, Lublin, Poland
4
Department of Anaesthesiological and Intensive Care Nursing, Medical University, Lublin, Poland
5
Department of Biochemistry and Molecular Biology, Medical University, Lublin, Poland
6
Department of Paediatric Neurology, Medical University, Lublin, Poland
Corresponding author
Jolanta Taczała
Department of Rehabilitation, Physiotherapy and Balneotherapy, Faculty of Health Sciences, Medical University of Lublin, Poland
Department of Rehabilitation, Physiotherapy and Balneotherapy, Faculty of Health Sciences, Medical University of Lublin, Poland
Ann Agric Environ Med. 2021;28(1):183-188
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Premature babies are a special group at risk of persistent brain damage caused by diseases, the most serious of which are cerebral palsy(CP), autism spectrum disorders (ASD) and mental retardation, among others. These conditions may occur concurrently, but appear more often as separate disease syndromes in the same group of at-risk children. Long-term observation of psychomotor development by an interdisciplinary medical team closely cooperating with parents is necessary. It is important to detect the risk of developing these diseases as soon as possible in all development spheres.
Material and methods:
The research was conducted to demonstrate the prognostic value of ‘red flags’ of developmental milestones and the ability to detect early signs of risk of developing CP and ASD in extremely premature babies. In this preliminary study, 42 preterm babies, born after less than 32 weeks pregnancy participated.
Results:
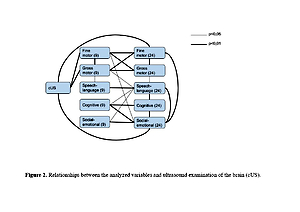
The occurrence of ‚red flags‘in the spheres: gross motor, fine motor and cognitive at 9 months was strongly associated with their presence at 24 months. The sensitivity and specificity were: gross motor – 0.91 (95% CI: 0.59, 1.00) and 0.94 (95% CI: 0.79, 0.99); fine motor – 0.83 (95% CI 0.36–1.00) and 1.00 (95% CI: 0.90–1.00); cognitive – 1.00 (0.40, 1.00) and 0.97 (0.86, 1.00). Other spheres had lower sensitivity but high specificity.
Conclusions:
The conclusion is that the ‚red flags‘at the 9 months milestones already predict the normal or developmental delay of premature babies, and predict the risk of CP and ASD. Due to the availability and lack of the need for specialized and costly training, it is worth considering their use in everyday life medical practice.
Premature babies are a special group at risk of persistent brain damage caused by diseases, the most serious of which are cerebral palsy(CP), autism spectrum disorders (ASD) and mental retardation, among others. These conditions may occur concurrently, but appear more often as separate disease syndromes in the same group of at-risk children. Long-term observation of psychomotor development by an interdisciplinary medical team closely cooperating with parents is necessary. It is important to detect the risk of developing these diseases as soon as possible in all development spheres.
Material and methods:
The research was conducted to demonstrate the prognostic value of ‘red flags’ of developmental milestones and the ability to detect early signs of risk of developing CP and ASD in extremely premature babies. In this preliminary study, 42 preterm babies, born after less than 32 weeks pregnancy participated.
Results:
The occurrence of ‚red flags‘in the spheres: gross motor, fine motor and cognitive at 9 months was strongly associated with their presence at 24 months. The sensitivity and specificity were: gross motor – 0.91 (95% CI: 0.59, 1.00) and 0.94 (95% CI: 0.79, 0.99); fine motor – 0.83 (95% CI 0.36–1.00) and 1.00 (95% CI: 0.90–1.00); cognitive – 1.00 (0.40, 1.00) and 0.97 (0.86, 1.00). Other spheres had lower sensitivity but high specificity.
Conclusions:
The conclusion is that the ‚red flags‘at the 9 months milestones already predict the normal or developmental delay of premature babies, and predict the risk of CP and ASD. Due to the availability and lack of the need for specialized and costly training, it is worth considering their use in everyday life medical practice.
REFERENCES (52)
1.
Duncan AF, Matthews MA. Neurodevelopmental Outcomes in Early Childchood. Clin Perinatol. 2018; 45: 377–392.
2.
Taczała J, Latalski M, Dmoszyńska-Graniczka M, Aftyka A, Majcher P. Neurodevelopmental outcome and early rehabilitation of premature babies – is it needed in the first 2 years of life? Ann Agric Environ Med. doi: 10.26444/aaem/122048.
3.
Smithers-Sheedy H, Badawi N, Blair E, Cans C, Himmelmann K, Krägeloh-Mann I, et al. What constitutes cerebral palsy in the twenty-first century? Dev Med Child Neurol. 2014; 56: 323–8.
4.
Novak I. Morgan C. Adde L, et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy Advances in Diagnosis and Treatment. Jama Pediatrics. 2017; 171(9): 897–907.
5.
Novak I, Hines M, Goldsmith S, et al. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics. 2012; 130(5), 1285–312.
6.
Cioni G, Inguaggiato E, Sgandurra G. Early intervention in neuro-developmental disorders: underlying neural mechanisms. Dev Med Child Neurol. 2016 Mac Keith Press, 58 (Suppl. 4): 61–66.
7.
Craciunoiu O, Holsti L. A Systematic Review of the Predictive Validity of Neurobehavioral Assessments During the Preterm Period. Physical & Occupational Therapy In Pediatrics, 2016; 37: 292–307.
8.
Herskind A, Greisen G, Nielsen JB. Early identification and intervention in cerebral palsy. Dev Med Child Neurol. 2015 Jan; 57(1): 29–36.
9.
Linsell L, Malouf R, Marlow N, et al. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. Jama Pediatr. 2015; 169(12): 1162–1172.
10.
Novak I. Mcintyre S. Morgan C, et al. A systematic review of interventions for children with cerebral palsy: state with the evidence. Dev Med Child Neur. 2013; 55: 885–910.
11.
Eeles A, Spittle A, Anderson P, Brown N, Lee K, Boyd R, Doyle L. Assessments of sensory processing in infants: a systematic review. Dev Med Child Neurol. 2013; 56: 314–326.
12.
Ting LH, Chiel HJ, Trumbower RD, McKay JC, Hackney ME, Kesar TM. Neuromechanical Principles Underlying Movement Modularity and Their Implications for Rehabilitation. Neuron. 2015; Apr 8, 86(1): 38–54.
13.
Himpens E, Van den Broeck C, Calders P, Vanhaesebruck P. Prevalence, type, distribution and severity of cerebral palsy in relations to gestational age: a metaanalytic review. Dev Med Child Neurol. 2008; 50(5): 334–40.
14.
Mottron L. Should we change targets and methods of early intervention in autism, in favor of a strengths-based education? Eur Child Adolesc Psychiatry 2017; 26: 815–825.
15.
Rutkowska M, Bekiesińska-Figatowska M, Kmita G, Terczyńska I, Polak K, Kalisiak M, Prażmowska D, Kiepura E, Szkudlińska-Pawlak S, Seroczyńska M, Helwich E. Neuroimaging results, short-term assessement of psychomotor development and the risk of autism spectrum disorder in extremly premature infants<28GA) – a prospective cohort study (preliminary report). Develop Peri Med. 2018; XXII(1): 39–48.
16.
Christensen D, Van Naarden Braun K, Doernberg N, Maenner MJ, Arneson CL, Durkin MS, Benedict RE, Kirby RS, Wingate MS, Fitzgerald R, Yeargin-Allsopp M. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA. Dev Med Child Neur. 2008; 56: 59–65.
17.
Hadders-Algra M. Early Diagnosis and Early Intervention Cerebral Palsy. Front Neurol Neuropediatrics. 2014; 5(185): 1–13.
18.
Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018 February; 30(1): 25–39.
19.
Johnson CP, Myers SM, American Academy of Pediatrics Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007; 120: 1183–215.
20.
WHO Motor Development Study: Windows of achievement for six gross motor development milestones. Acta Pediatrica 2006; Suppl 450: 86–95.
21.
Dosman CF, Andrews D, Goulden K. Evidence-based milestone ages as a framework for developmental surveillance. Paediatr Child Health 2012; 17: 561–568.
22.
Jarjour IT. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol. 2015; 52(2): 143–52.
23.
Towle P, Patrick P. Autism Spectrum Disorder Screening Instruments for Very Young Children: A Systematic Review. Autism Research and Treatment, 2016, Article ID 4624829, p.29.
24.
Pineda R, Melchior K, Oberle S, Inder T, Rogers C. Assessment of Autism Symptoms During the Neonatal Period: Is There Early Evidence of Autism Risk? Am J Occup Therapy. 2015; 69(4): 6904220010.
25.
Moore T, Hennessy E, Myles J, et al. Neurological and developmental outcome in extremly preterm children born in England in 2005 and 2006: the EPICure studies. BMJ 2012; 345: 1–15.
26.
Ancel P-Y, Goffined F and EPIPAGE -2 Writing Group. Survival and Morbidity of Preterm Children born et 22 Through 34 Week’s Gestation in France in 2011. Results of EPIPAGE-2 Cohort Study. JAMA Pediatr. 2015; 169(3): 230–238.
27.
Sturner R, Howard B, Bergmann P, Morrel T, Andon LD, Rao P, Landa R. Autism Screening With Online Decision Support by Primary Care Pediatricians Aided by M-CHAT/F. Pediatrics. 2016; 138 (3): e 20153036.
28.
Kim SH, Joseph RM, Frazier JA, O’Shea TM, Chawarska K, Allred EN, Leviton A, Kuban KK. SMEpih on behalf of the Extremely Low Gestational Age Newborn (ELGAN) Study Investigators: Predictive Validity of the Modified Checklist for Autism in Toddlers (M-CHAT) Born Very Preterm. J Pediatr. 2016 November; 178: 101–107.
29.
Magán-Maganto M, Bejarano-Martín A, Fernández-Alvarez C, Narzisi A, García-Primo P, Kawa R, Posada M, Canal-Bedia R. Early Detection and Intervention of ASD: A European Overview. Brain Sci. 2017; Vol. 7: 159.
30.
Dudova I, Kasparova M, Markova D, et al. Screening for autism in preterm children with extremely low and very low birth weight. Neuropsychiatr Dis Treat. 2014; 10: 277–282.
31.
Van Eyk C, Corbett MA, Gardner A, van Bon BW, Broadbent JL, Harper K, MacLennan AH, Gecz J. Analysis of 182 cerebral palsy transcriptomes points to dysregulation of trophic signalling pathways and overlap with autism. Translational Psychiatry 2018; 8: 88.
32.
Maenner M, Blumberg S, Kogan M, PhDd, Christensen D, Yeargin-Allsopp M, Schieve L: Prevalence of cerebral palsy and intellectual disability among children identified in two U.S. National Surveys, 2011–2013. Ann Epidemiol. 2016 March; 26(3): 222–226.
33.
Spittle AJ, Olsen J, Kwong A, Doyle LW, Marschik PB, Einspieler C, Cheong JLY. The Baby Moves prospective cohort study protocol: using a smartphone application with the General Movements Assessment to predict neurodevelopmental outcomes at age 2 years for extremely preterm or extremely low birthweight infants. BMJ Open 2016; 6: e013446.
34.
Einspieler C, Marschik PB, Bos AF, Ferrari F, Cioni G, Prechtl HF. Early markers for cerebral palsy: insights from the assessment of general movements. Future Neurol. 2012; 7: 709–717.
35.
Romeo D, Cioni M, Palermo F, Cilauro S, Romeo M. Neurological Assessment in infants discharged from a neonatal intensive care unit. Eur J Ped Neurol. 2013; 17: 192–198.
36.
Bosanquet M, Copeland L, Ware R, Boyd R. A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol. 2013; 55: 418–26.
37.
Torres EB, Smith B, MistryS, Brincker M, Whyat C. Neonatal Diagnostics: Toward Dynamic Growth Charts of Neuromotor Control. Front Pediatr. 2016; 4: 121.
38.
Piresa C, Marbaa S, Caldasa J, Sanchez Stopigliaa M. Predictive value of the general movements assessement in preterm infants: a meta-analysis. Rev Paul Pediatr. 2020; 38: e2018286.
39.
Spittle AJ, Morgan C, Olsen JE, Novak I, Cheong JL. Early Diagnosis and Treatment of Cerebral Palsy in Cildren with a History of Preterm Birth. Clin Perinatol. 2018; 45(3): 409–420.
40.
Morgan C, Romeo DM, Chorna O, Novak I, Galea C, Secco S, Guzzetta A: The Pooled Diagnostic Accuracy of Neuroimaging, General Movements, and Neurological Examination for Diagnosing Cerebral Palsy Early in High-Risk Infants: A Case Control Study. J Clin Med. 2019; 8: 1879.
41.
Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatrics 2006; 118: 405–20.
42.
Shepherd E, Salam RA, Middleton P, Han S, Makrides M, McIntyre S, Badawi N, Crowther CA. Neonatal interventions for preventing cerebral palsy: an overview of Cochrane Systematic Reviews (Review). Cochrane Database of Systematic Reviews 2018; 6. Art. No.: CD012409.
43.
Morgan C, Darrah J, Gordon AM, Harbourne R, Spittle A, Johnson R, Fetters L. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Chil Neur. 2016; 58: 900–909.
44.
Moore T, Hennessy E, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 2012; 345: 1–15.
45.
Constantino J, Marrus N. The Early Origins of Autism. Child Adolesc Psychiatric Clin N Am. 2017; 26: 555–570.
46.
Diwakar RK, Khurana O. Cranial Sonography in Preterm Infants with Short Review of Literature. J Ped Neurosci. 2018; 13(2): 141–149.
47.
Maitre NL, Slaughter JC, Aschner JL. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev. 2013; 89: 781–6.
48.
Velde A, Morgan C, Novak I, Tantsis E, Badawi N. Early Diagnosis and Classification of Cerebral Palsy: An Historical Perspective and Barriers to an Early Diagnosis. J Clin Med. 2019; 8: 1599.
49.
Benzies KM, Magill-Evans JE, Hayden KA, et al. Key components of early intervention programs for preterm infants and their parents. A systematic reviews and meta-analysis. BMC Pregnancy Childbirth 2013; 13(supl 1): 10.
50.
Novak I, Thornton N, Morgan M, et al. Truth with hope: ethical challenges in disclosing „bad” diagnostic prognostic and intervention information. In: Rosenbaum P, Ronen GM, Racine E, et al. editors Ethic in child health: principles and cases in neurodisability. London MacKeith Press 2016.
51.
Di Rossa G, Cavallaro T, Alibrandi A, et al. Predictive role of milestones-related psychomotor profiles and long-term neurodevelopmental pitfalls in preterm infants. Early Hum Dev. 2016; 101: 49–55.
52.
Noble Y, Boyd R. Neonatal assessements for the preterm infant up to four months corrected age: a systematic review. Dev Med Child Neur. 2012; 54: 129–139.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.