Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
The influence of lithium and/or selenium on plasma amino acids profile in rats
1
Chair and Department of Medical Chemistry, Medical University, Lublin, Poland
2
Department of Virology with SARS Laboratory, Medical University, Lublin, Poland
Corresponding author
Małgorzata Kiełczykowska
Chair and Department of Medical Chemistry, Medical University of Lublin, Chodźki 4 a, 20-093, Lublin, Poland
Chair and Department of Medical Chemistry, Medical University of Lublin, Chodźki 4 a, 20-093, Lublin, Poland
Ann Agric Environ Med. 2022;29(1):136-142
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Selenium belongs to essential microelements and is used in agriculture. Lithium is used in medicine and the possibility of its exposure by environmental pollution has been reported. Both elements have been found to be connected with amino acids metabolism.
Objective:
The aim of the study was to compare the effect of lithium and selenium on plasma amino acids in rats, and to evaluate the influence of selenium in organisms exposed to lithium.
Material and methods:
The effect of selenium (0.5 mg/kg b.w., orally as Na2SeO3) and/or lithium (2.7 mg/kg b.w., orally as Li2CO3) given for 6 weeks on the plasma profile of selected amino acids in rats was studied. The concentrations of amino acids were determined using ion exchange chromatography with the aid of an amino acids analyzer AAA400.
Results:
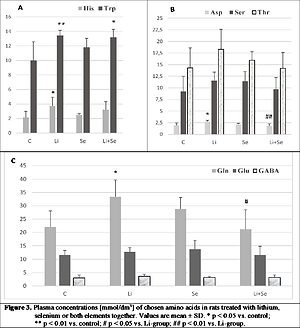
A significant effect of lithium on plasma amino acids profile was found in rats, much greater than for selenium. Selenium treatment slightly increased Tau, Phe, Tyr, Ala, Trp, Ser and Gln, while Lys and Orn were enhanced in a significant way. In contrast, Li-treatment caused a well-marked increase in Phe, Orn, Ala, His, Trp, Asp and Gln, whereas all the others were only slightly increased. Co-treatment resulted in a significant increase in Orn and Trp, a slight enhancement of Phe, Lys and His, while the rest remained unchanged.
Conclusions:
A significant effect of lithium alone on plasma amino acids profile in animals was demonstrated, with a much less influence of selenium alone. Co-treatment generally resulted in a slight or no effect. The slight selenium influence seems important regarding its agricultural application and the growing interest in its supplementation. Results concerning lithium could contribute to the research regarding the mechanism of Li action.
Selenium belongs to essential microelements and is used in agriculture. Lithium is used in medicine and the possibility of its exposure by environmental pollution has been reported. Both elements have been found to be connected with amino acids metabolism.
Objective:
The aim of the study was to compare the effect of lithium and selenium on plasma amino acids in rats, and to evaluate the influence of selenium in organisms exposed to lithium.
Material and methods:
The effect of selenium (0.5 mg/kg b.w., orally as Na2SeO3) and/or lithium (2.7 mg/kg b.w., orally as Li2CO3) given for 6 weeks on the plasma profile of selected amino acids in rats was studied. The concentrations of amino acids were determined using ion exchange chromatography with the aid of an amino acids analyzer AAA400.
Results:
A significant effect of lithium on plasma amino acids profile was found in rats, much greater than for selenium. Selenium treatment slightly increased Tau, Phe, Tyr, Ala, Trp, Ser and Gln, while Lys and Orn were enhanced in a significant way. In contrast, Li-treatment caused a well-marked increase in Phe, Orn, Ala, His, Trp, Asp and Gln, whereas all the others were only slightly increased. Co-treatment resulted in a significant increase in Orn and Trp, a slight enhancement of Phe, Lys and His, while the rest remained unchanged.
Conclusions:
A significant effect of lithium alone on plasma amino acids profile in animals was demonstrated, with a much less influence of selenium alone. Co-treatment generally resulted in a slight or no effect. The slight selenium influence seems important regarding its agricultural application and the growing interest in its supplementation. Results concerning lithium could contribute to the research regarding the mechanism of Li action.
ABBREVIATIONS
Met – methionine; Tau – taurine, Phe – phenylalanine; Tyr – tyrosine; Lys – lysine; Orn – ornithine; Gly – glycine; Ala –alanine; His – histidine; Trp – tryptophan; Asp – aspartate, Ser – serine; Thr – threonine; Gln – glutamine; Glu – glutamate; GABA – g-aminobutyric acid; ALT – alanine amino transferase; AST – aspartate amino transferase
REFERENCES (49)
1.
Trippe RC 3rd, Pilon-Smits EAH. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J Hazard Mater. 2021; 404(Pt B): 124178. https://doi.org/10.1016/j.jhaz....
2.
Kieliszek M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules. 2019; 24(7): 1298. doi: 10.3390/molecules24071298.
3.
Andrade FR, da Silva GN, Guimaraes KC, et al. Selenium protects rice plants from water deficit stress. Ecotoxicol Environ Saf. 2018; 164: 562–570. doi: 10.1016/j.ecoenv.2018.08.022. https://doi.org/10.1016/j.ecoe....
4.
Jóźwiak W, Politycka B. Effect of Selenium on Alleviating Oxidative Stress Caused by a Water Deficit in Cucumber Roots. Plants (Basel). 2019; 8(7): 217. doi: 10.3390/plants8070217. https://doi.org/10.3390/plants....
5.
Rayman MP. Selenium intake, status, and health: a complex relationship. Hormones (Athens). 2020; 19(1): 9–14. doi: 10.1007/s42000-019-00125-5.
6.
Gangadoo S, Dinev I, Willson NL, et al. Nanoparticles of selenium as high bioavailable and non-toxic supplement alternatives for broiler chickens. Environ Sci Pollut Res Int. 2020; 27(14): 16159–16166. doi: 10.1007/s11356-020-07962-7.
7.
Liang N, Wang F, Peng X, et al. Effect of Sodium Selenite on Pathological Changes and Renal Functions in Broilers Fed a Diet Containing Aflatoxin B1. Int J Environ Res Public Health. 2015; 12(9): 11196–11208. https://doi.org/10.3390/ijerph....
8.
Ramkissoon C, Degryse F, da Silva RC, et al. Improving the efficacy of selenium fertilizers for wheat biofortification. Sci Rep. 2019; 9(1): 19520. doi: 10.1038/s41598-019-55914-0. Erratum in: Sci Rep. 2020; 10(1): 16963. https://doi.org/10.1038/s41598....
9.
Mehta SQ, Behl S, Day PL, et al. Evaluation of Zn, Cu, and Se Levels in the North American Autism Spectrum Disorder Population. Front Mol Neurosci. 2021; 14: 665686. https://doi.org/10.3389/fnmol.....
10.
Scassellati C, Bonvicini C, Benussi L, et al. Neurodevelopmental disorders: Metallomics studies for the identification of potential biomarkers associated to diagnosis and treatment. J Trace Elem Med Biol. 2020; 60: 126499. https://doi.org/10.1016/j.jtem....
11.
Constantinescu-Aruxandei D, Frîncu RM, Capră L, et al. Selenium Analysis and Speciation in Dietary Supplements Based on Next-Generation Selenium Ingredients. Nutrients. 2018; 10(10): 1466. doi: 10.3390/nu10101466.
12.
Qu KC, Li HQ, Tang KK, et al. Selenium Mitigates Cadmium-Induced Adverse Effects on Trace Elements and Amino Acids Profiles in Chicken Pectoral Muscles. Biol Trace Elem Res. 2020; 193(1): 234–240. https://doi.org/10.1007/s12011....
13.
Rasinger JD, Lundebye AK, Penglase SJ, et al. Methylmercury Induced Neurotoxicity and the Influence of Selenium in the Brains of Adult Zebrafish (Danio rerio). Int J Mol Sci. 2017; 18(4): 725. https://doi.org/10.3390/ijms18....
14.
Solovyev ND. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J Inorg Biochem. 2015; 153: 1–12. https://doi.org/10.1016/j.jino....
15.
Antoniadou I, Kouskou M, Arsiwala T, et al. Ebselen has lithium-like effects on central 5-HT2A receptor function. Br J Pharmacol. 2018; 175(13): 2599–2610. https://doi.org/10.1111/bph.14....
16.
Masaki C, Sharpley AL, Godlewska BR, et al. Effects of the potential lithium-mimetic, ebselen, on brain neurochemistry: a magnetic resonance spectroscopy study at 7 tesla. Psychopharmacology (Berl). 2016; 233(6): 1097–1104. doi: 10.1007/s00213-015-4189-2.
17.
Masaki C, Sharpley AL, Cooper CM, et al. Effects of the potential lithium-mimetic, ebselen, on impulsivity and emotional processing. Psychopharmacology (Berl). 2016; 233(14): 2655–2661. doi: 10.1007/s00213-016-4319-5.
18.
Boga Pekmezekmek A, Emre M, Tunc E, et al. L-Glutamic acid monosodium salt reduces the harmful effect of lithium on the development of Xenopus laevis embryos. Environ Sci Pollut Res Int. 2020; 27(33): 42124–42132. https://doi.org/10.1007/s11356....
19.
Rybakowski JK. Lithium treatment in the era of personalized medicine. Drug Dev Res. 2020; https://doi.org/10.1002/ddr.21....
20.
McFarlane HG, Steele J, Vinion K, et al. Acute lithium administration selectively lowers tyrosine levels in serum and brain. Brain Res. 2011; 1420: 29–36. doi: 10.1016/j.brainres.2011.08.054.
21.
Farooq M, Kim S, Patel S, et al. Lithium increases synaptic GluA2 in hippocampal neurons by elevating the ?-catenin protein. Neuropharmacology. 2017; 113(Pt A): 426–433. https://doi.org/10.1016/j.neur....
22.
Joshi MB, Pai S, Balakrishnan A, et al. Evidence for perturbed metabolic patterns in bipolar disorder subjects associated with lithium responsiveness. Psychiatry Res. 2019; 273: 252–259. https://doi.org/10.1016/j.psyc....
23.
Wakita M, Nagami H, Takase Y, et al. Modifications of excitatory and inhibitory transmission in rat hippocampal pyramidal neurons by acute lithium treatment. Brain Res Bull. 2015; 117: 39–44. doi: 10.1016/j.brainresbull.2015.07.009.
24.
Kubo H, Nakataki M, Sumitani S, et al. 1H-magnetic resonance spectroscopy study of glutamate-related abnormality in bipolar disorder. J Affect Disord. 2017; 208: 139–144. https://doi.org/10.1016/j.jad.....
25.
Sarawagi A, Soni ND, Patel AB. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder. Front Psychiatry. 2021;12: 637863. https://doi.org/10.3389/fpsyt.....
26.
Teo CH, Soga T, Parhar I. Lithium chloride enhances serotonin induced calcium activity in EGFP-GnIH neurons. Sci Rep. 2020; 10(1): 13876. https://doi.org/10.1038/s41598....
27.
An Y, Inoue T, Kitaichi Y, et al. Combined treatment with subchronic lithium and acute intracerebral mirtazapine microinjection into the median raphe nucleus exerted an anxiolytic-like effect synergistically. Eur J Pharmacol. 2016; 783: 112–116. https://doi.org/10.1016/j.ejph....
28.
Harari F, Bottai M, Casimiro E, et al. Exposure to Lithium and Cesium Through Drinking Water and Thyroid Function During Pregnancy: A Prospective Cohort Study. Thyroid. 2015; 25(11): 1199–1208. doi: 10.1089/thy.2015.0280.
29.
Meshram P, Mishra A, Abhilash, et al. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids – A review. Chemosphere. 2020; 242: 125291. doi: 10.1016/j.chemosphere.2019.125291.
30.
Leenaars CHC, Drinkenburg WHP, Nolten C, et al. Sleep and Microdialysis: An Experiment and a Systematic Review of Histamine and Several Amino Acids. J Circadian Rhythms. 2019; 17: 7. https://doi.org/10.5334/jcr.18....
31.
Shah PA, Park CJ, Shaughnessy MP, et al. Serotonin as a Mitogen in the Gastrointestinal Tract: Revisiting a Familiar Molecule in a New Role. Cell Mol Gastroenterol Hepatol. 2021: S2352-345X(21)00097-7. https://doi.org/10.1016/j.jcmg....
32.
Dinu A, Apetrei C. A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis. Sensors (Basel). 2020; 20(9): 2496. doi: 10.3390/s20092496.
33.
Moriguchi T, Takai J. Histamine and histidine decarboxylase: Immunomodulatory functions and regulatory mechanisms. Genes Cells. 2020; 25(7): 443–449. https://doi.org/10.1111/gtc.12....
34.
Zhou L, Feng Y, Liu Y, et al. Serine Supplementation in the Diets of Late Gestating and Lactating Sows Improves Selenium Nutritional Status in Sows and Their Offspring. Biol Trace Elem Res. 2021; https://doi.org/10.1007/s12011....
35.
Ben Saad A, Dalel B, Rjeibi I, et al. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharm Biol. 2017; 55(1): 516–525. https://doi.org/10.1080/138802....
36.
Venn BJ, Grant AM, Thomson CD, et al. Selenium supplements do not increase plasma total homocysteine concentrations in men and women. J Nutr. 2003; 133(2): 418–420. https://doi.org/10.1093/jn/133....
37.
González S, Huerta JM, Alvarez-Uría J, et al. Serum selenium is associated with plasma homocysteine concentrations in elderly humans. J Nutr. 2004; 134(7): 1736–1740. https://doi.org/10.1093/jn/134....
38.
Rey AI, de-Cara A, Calvo L, et al. Changes in Plasma Fatty Acids, Free Amino Acids, Antioxidant Defense, and Physiological Stress by Oleuropein Supplementation in Pigs Prior to Slaughter. Antioxidants (Basel). 2020; 9(1): 56. https://doi.org/10.3390/antiox....
39.
Yang CJ, Huang TS, Lee TL, et al. Serum Glutamine Levels as a Potential Diagnostic Biomarker in Sepsis following Surgery for Peritonitis. Chin J Physiol. 2017; 60(6): 320–326. doi: 10.4077/CJP.2017.BAG495.
40.
Jang JY, Lee SH, Shim H, et al. Serum oxygen radical activity and total antioxidation capacity are related with severities of surgical patient with sepsis: Prospective pilot study. J Crit Care. 2017; 39: 131–136. https://doi.org/10.1016/j.jcrc....
41.
Sabatino BR, Rohrbach BW, Armstrong PJ, et al. Amino acid, iodine, selenium, and coat color status among hyperthyroid, Siamese, and age-matched control cats. J Vet Intern Med. 2013; 27(5): 1049–1055. https://doi.org/10.1111/jvim.1....
42.
Al-Quraishy S, Dkhil MA, Abdel Moneim AE. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine. 2015; 10: 6741–6756. https://doi.org/10.2147/IJN.S9....
43.
Ansar S, Alshehri SM, Abudawood M, et al. Antioxidant and hepatoprotective role of selenium against silver nanoparticles. Int J Nanomedicine. 2017; 12: 7789–7797. https://doi.org/10.2147/IJN.S1.... Erratum in: Int J Nanomedicine. 2018; 13: 5769.
44.
Pettegrew JW, Panchalingam K, McClure RJ, et al. Effects of chronic lithium administration on rat brain phosphatidylinositol cycle constituents, membrane phospholipids and amino acids. Bipolar Disord. 2001; 3(4): 189–201. https://doi.org/10.1034/j.1399....
45.
Bhalla P, Singla N, Dhawan DK. Potential of lithium to reduce aluminium-induced cytotoxic effects in rat brain. Biometals. 2010; 23(2): 197–206. doi: 10.1007/s10534-009-9278-4.
46.
Friedman SD, Dager SR, Parow A, et al. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry. 2004; 56(5): 340–348. https://doi.org/10.1016/j.biop....
47.
Zanetti MV, Otaduy MC, de Sousa RT, et al. Bimodal effect of lithium plasma levels on hippocampal glutamate concentrations in bipolar II depression: a pilot study. Int J Neuropsychopharmacol. 2014; 18(6): pyu058. doi: 10.1093/ijnp/pyu058.
48.
Park J, Cheon W, Kim K. Effects of Long-Term Endurance Exercise and Lithium Treatment on Neuroprotective Factors in Hippocampus of Obese Rats. Int J Environ Res Public Health. 2020; 17(9): 3317. https://doi.org/10.3390/ijerph....
49.
Davis J, Desmond M, Berk M. Lithium and nephrotoxicity: Unravelling the complex pathophysiological threads of the lightest metal. Nephrology (Carlton). 2018; 23(10): 897–903. https://doi.org/10.1111/nep.13....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.