Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Single-strand conformation polymorphism-based genetic characterization of the Cyclospora cayetanensis strains collected from different provinces in Turkey
1
Department of Parasitology, Faculty of Medicine, Kırşehir Ahi Evran University, Turkey
2
Department of Genetic, Faculty of Veterinary Medicine, Dicle University, Turkey
3
Department of Parasitology, Faculty of Medicine, Yuzuncu Yil University, Turkey
4
Department of Parasitology, Faculty Medicine, Ordu University, Turkey
Corresponding author
Muttalip Cicek
Kırşehir Ahi Evran University, Faculty of Medicine, Department of Parasitology, Kırşehir Ahi Evran Üniversitesi Tıp Fakültesi Deka, 40100, Kırşehir, Turkey
Kırşehir Ahi Evran University, Faculty of Medicine, Department of Parasitology, Kırşehir Ahi Evran Üniversitesi Tıp Fakültesi Deka, 40100, Kırşehir, Turkey
Ann Agric Environ Med. 2021;28(2):267-270
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
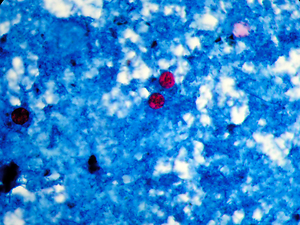
Cyclospora cayetanensis, a coccidian protozoan species, has been recently found to cause diarrhea in all age groups in immunocompetent and immunocompromised individuals in most regions of the world. This study aimed to conduct the molecular detection of C. cayetanensis and to determine the genetic diversity of the 18S ribosomal RNA (rRNA) gene sequence of C. cayetanensis isolated from individuals living in different provinces in Turkey by using PCR–single-strand conformation polymorphism (SSCP).
Material and methods:
A total of 22 subjects were included in the study. Fourteen of the subjects were female and eight were male, with ages ranging between 7–65 years. Stool specimens were examined using wet mount and modified acid-fast staining methods, which revealed the presence of oocysts in the samples. The 18S rRNA ITS-1 Ccits37f-GCTTGCTATGTTTTAGCATGTGG and Ccits501r-GCACAATGAATGCACACACA gene regions were used as primers. The PCR products were analyzed by agarose gel electrophoresis and visualized on a UV transilluminator. For the SSCP, the PCR products were denatured with formamide, run for 16 h in 6% (49:1) polyacrylamide gel, and then imaged with silver staining.
Results:
SSCP assay was performed given that the DNA strands demonstrated different folds; the DNA strands contain different nucleotides based on the PCR-SSCP results for the Cyclospora strains collected in 4 provinces. Moreover, 3 different band profiles were observed in the investigated samples. A slight mutation difference was observed among the strains collected.
Conclusions:
Further comprehensive studies involving more C. cayetanensis-positive specimens and utilizing different mutation screening methods are warranted to demonstrate mutation differences in Cyclopora strains in Turkey.
Cyclospora cayetanensis, a coccidian protozoan species, has been recently found to cause diarrhea in all age groups in immunocompetent and immunocompromised individuals in most regions of the world. This study aimed to conduct the molecular detection of C. cayetanensis and to determine the genetic diversity of the 18S ribosomal RNA (rRNA) gene sequence of C. cayetanensis isolated from individuals living in different provinces in Turkey by using PCR–single-strand conformation polymorphism (SSCP).
Material and methods:
A total of 22 subjects were included in the study. Fourteen of the subjects were female and eight were male, with ages ranging between 7–65 years. Stool specimens were examined using wet mount and modified acid-fast staining methods, which revealed the presence of oocysts in the samples. The 18S rRNA ITS-1 Ccits37f-GCTTGCTATGTTTTAGCATGTGG and Ccits501r-GCACAATGAATGCACACACA gene regions were used as primers. The PCR products were analyzed by agarose gel electrophoresis and visualized on a UV transilluminator. For the SSCP, the PCR products were denatured with formamide, run for 16 h in 6% (49:1) polyacrylamide gel, and then imaged with silver staining.
Results:
SSCP assay was performed given that the DNA strands demonstrated different folds; the DNA strands contain different nucleotides based on the PCR-SSCP results for the Cyclospora strains collected in 4 provinces. Moreover, 3 different band profiles were observed in the investigated samples. A slight mutation difference was observed among the strains collected.
Conclusions:
Further comprehensive studies involving more C. cayetanensis-positive specimens and utilizing different mutation screening methods are warranted to demonstrate mutation differences in Cyclopora strains in Turkey.
ACKNOWLEDGEMENTS
The study was supported by Dicle University in Diyarbakır,
Turkey, Scientific Research Coordinatorship (DÜBAP),
Project No. 11-TF-58.
REFERENCES (22)
1.
Cicek M, Palanci Y, Ozekinci ACT, et al. Evaluation of demographic, clinic and treatment features of patients and a cross-sectional survey of cyclosporiasis in patients with diarrhea in Southeastern Turkey. Afr J Microbiol Res. 2012; 6(12): 2949–2955.
2.
Hadjilouka A, Tsaltas D. Cyclospora Cayetanensis-Major Outbreaks from Ready to Eat Fresh Fruits and Vegetables. Foods (Basel, Switzerland). 2020; 9(11).
3.
Siwila J, Mwaba F, Chidumayo N, et al. Food and waterborne protozoan parasites: The African perspective. Food Waterborne Parasitol. 2020; 20: e00088.
4.
Ortega YR, Sanchez R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin Microbiol Rev. 2010; 23(1): 218–234.
5.
Hall RL, Jones JL, Herwaldt BL. Surveillance for laboratory-confirmed sporadic cases of cyclosporiasis--United States, 1997–2008. MMWR Surveill Summ. 2011; 60(2): 1–11.
6.
Ma DW, Lee MR, Ku B, et al. Outbreak of Cyclosporiasis in Korean Travelers Returning from Nepal. Korean J Parasitol. 2020; 58(5): 589–592.
7.
Eberhard ML, Owens JR, Bishop HS, et al. Cyclospora spp. in drills, Bioko Island, Equatorial Guinea. Emerg Infect Dis. 2014; 20(3): 510–511.
8.
Zhao GH, Cong MM, Bian QQ, et al. Molecular characterization of Cyclospora-like organisms from golden snub-nosed monkeys in Qinling Mountain in Shaanxi province, northwestern China. PLoS One. 2013; 8(2): e58216.
9.
Sulaiman IM, Torres P, Simpson S, et al. Sequence characterization of heat shock protein gene of Cyclospora cayetanensis isolates from Nepal, Mexico, and Peru. J Parasitol. 2013; 99(2): 379–382.
10.
Cinar HN, Gopinath G, Jarvis K, et al. The Complete Mitochondrial Genome of the Foodborne Parasitic Pathogen Cyclospora cayetanensis. PLoS One. 2015; 10(6): e0128645.
11.
Cinar HN, Gopinath G, Murphy HR, et al. Molecular typing of Cyclospora cayetanensis in produce and clinical samples using targeted enrichment of complete mitochondrial genomes and next-generation sequencing. Parasit Vectors. 2020; 13(1): 122.
12.
Özkara H. Mutasyon Tarama Yöntemleri. J Türk Klinik Biyokimya Dergi. 2003; 1: 47–53.
13.
Nataraj AJ, Olivos-Glander I, Kusukawa N, et al. Single-strand conformation polymorphism and heteroduplex analysis for gel-based mutation detection. Electrophoresis. 1999; 20(6): 1177–1185.
14.
Lalonde LF, Gajadhar AA. Highly sensitive and specific PCR assay for reliable detection of Cyclospora cayetanensis oocysts. Appl Environ Microbiol. 2008; 74(14): 4354–4358.
15.
Büget E, Boral OB, Uysal HK, et al. Case report: C. cayetanensis diarrhea was firstly documented in Turkey. Türk Mikrobiyoloji Society Journal. 2000; 30: 162–165.
16.
Turgay N, Yolasigmaz A, Erdogan DD, et al. Incidence of cyclosporiasis in patients with gastrointestinal symptoms in western Turkey. Med Sci Monit. 2007; 13(1): CR34–39.
17.
Karaman U, Daldal N, Ozer A, et al. Epidemiology of Cyclospora Species in Humans in Malatya Province in Turkey. Jundishapur J Microbiol. 2015; 8(7): e18661.
18.
Tas Cengiz Z, Beyhan YE, Yilmaz H. Cyclospora cayetanensis, Opportunistic Protozoan Parasite, in Van Province, Turkey: A Report of Seven Cases. Turkiye Parazitol Derg. 2016; 40(3): 166–168.
19.
Ozdamar M, Hakko E, Turkoglu S. High occurrence of cyclosporiasis in Istanbul, Turkey, during a dry and warm summer. Parasit Vectors. 2010; 3: 39.
20.
Yadav P, Khalil S, Mirdha BR, et al. Molecular characterization of clinical isolates of Cyclospora cayetanensis from patients with diarrhea in India. Indian J Med Microbiol. 2015; 33(3): 351–356.
21.
Sulaiman IM, Ortega Y, Simpson S, et al. Genetic characterization of human-pathogenic Cyclospora cayetanensis parasites from three endemic regions at the 18S ribosomal RNA locus. Infect Genet Evol. 2014; 22: 229–234.
22.
Hussein EM, El-Moamly AA, Mahmoud MA, et al. Wide genetic variations at 18S ribosomal RNA locus of Cyclospora cayetanensis isolated from Egyptian patients using high resolution melting curve. Parasitol Res. 2016; 115(7): 2797–2806.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.