Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Role of diet in primary and secondary prevention of periodontitis and non-specific inflammatory bowel diseases. Part II
1
Institute of Rural Health, Lublin, Poland
2
Student of the Faculty of Medicine and Dentistry, Jagiellonian University Medical College, Kraków, Poland
3
Higher School of Health Promotion, Kraków, Poland
Corresponding author
Małgorzata Goździewska
Institute of Rural Health, Lublin, Poland, Jaczewskiego, 20-090, Lublin, Poland
Institute of Rural Health, Lublin, Poland, Jaczewskiego, 20-090, Lublin, Poland
Ann Agric Environ Med. 2024;31(2):170-177
KEYWORDS
inflammatory bowel diseasesdiet nutritional recommendationsgut microfloraoral microfloraperiodontal diseases
TOPICS
ABSTRACT
Introduction and objective:
Both periodontitis and non-specific bowel diseases (IBD) are complex chronic diseases, and the elements connecting them are the dysregulated microbiota and abnormal immune response of the host. In turn, in the etiology of these diseases, the common environmental risk factor is improper mode of nutrition. The aim of the study is to review nutritional interventions and effective nutritional protocols applied in periodontitis and IBD. The result of the review will be identification of dietary recommendations exerting a beneficial effect on the reduction of the risk of development and alleviation of the severity of both diseases. At the same time, non-recommended dietary choices will be indicated.
Review methods:
A review of literature was carried out using the databases PubMed, Google Scholar, and Web of Science. Publications were analyzed by a non-systematic literature review aimed at making a brief synthesis of the collected information.
Brief description of the state of knowledge:
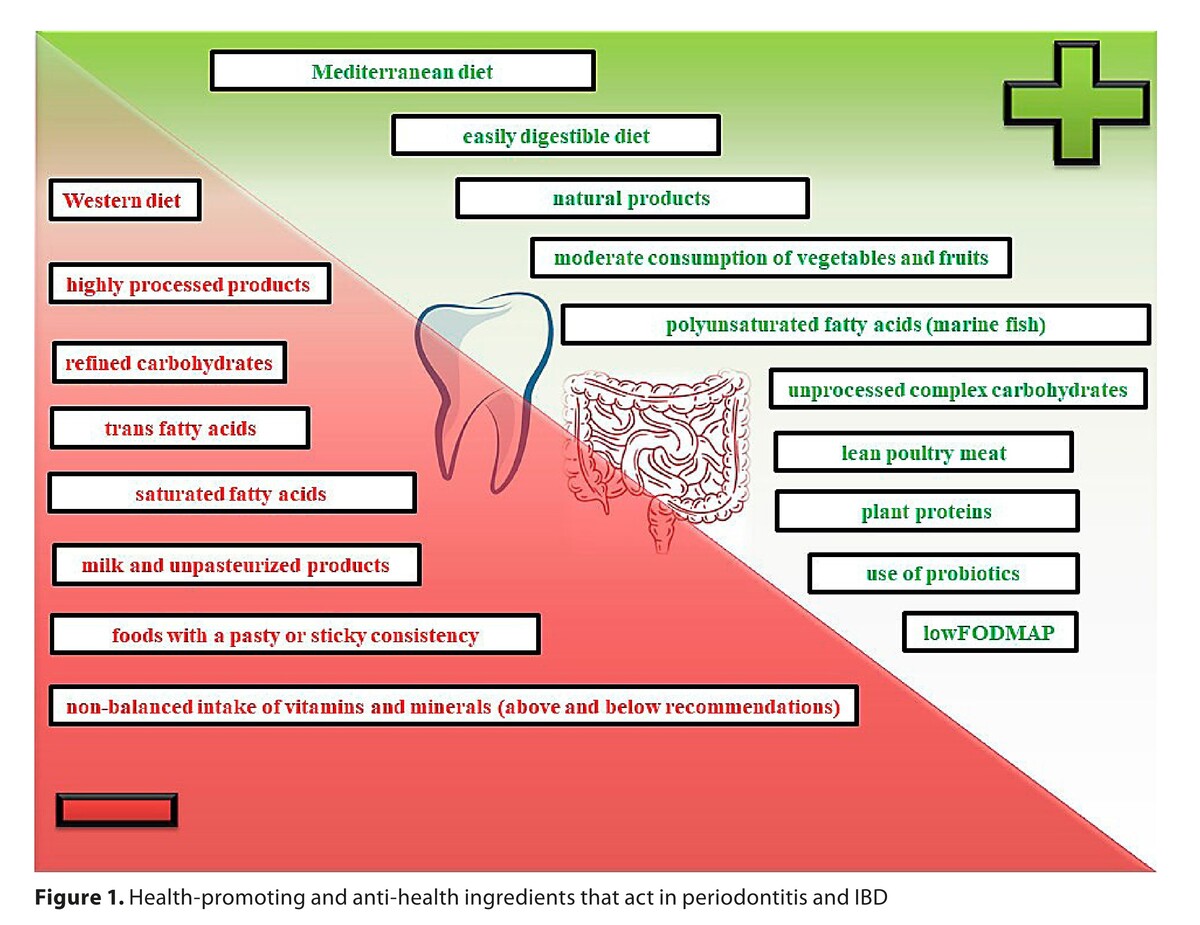
Diets recommended to patients with both periodontitis and IBD included the Mediterranean diet, DASH diet and vegetarian diet; excluding veganism, raw foodism and fruitarianism. For patients with IBD, special dietary recommendations were elaborated on the recommendations of the International Organization for Research into Inflammatory Bowel Diseases (IOIBD), and specific diets, i.e. specific carbohydrate diet (SCD), and Groningen anti-inflammatory diet (GrAID). In the process of treatment of oral and intestinal dysbiosis, probiotic therapy is beneficial in both diseases, specified as the Western diet. Non-conventional diets are not recommended.
Summary:
Diet therapy for inflammatory periodontal diseases and IBD requires extensive individualization; nevertheless, a universal principle is avoidance of highly processed food, and implementation of easily digestible meals based on natural, ecological products. Proper nutrition plays a crucial role in primary prevention of both diseases analyzed, whereas in secondary prevention, diet therapy is a valuable supplementation of pharmacotherapy.
Both periodontitis and non-specific bowel diseases (IBD) are complex chronic diseases, and the elements connecting them are the dysregulated microbiota and abnormal immune response of the host. In turn, in the etiology of these diseases, the common environmental risk factor is improper mode of nutrition. The aim of the study is to review nutritional interventions and effective nutritional protocols applied in periodontitis and IBD. The result of the review will be identification of dietary recommendations exerting a beneficial effect on the reduction of the risk of development and alleviation of the severity of both diseases. At the same time, non-recommended dietary choices will be indicated.
Review methods:
A review of literature was carried out using the databases PubMed, Google Scholar, and Web of Science. Publications were analyzed by a non-systematic literature review aimed at making a brief synthesis of the collected information.
Brief description of the state of knowledge:
Diets recommended to patients with both periodontitis and IBD included the Mediterranean diet, DASH diet and vegetarian diet; excluding veganism, raw foodism and fruitarianism. For patients with IBD, special dietary recommendations were elaborated on the recommendations of the International Organization for Research into Inflammatory Bowel Diseases (IOIBD), and specific diets, i.e. specific carbohydrate diet (SCD), and Groningen anti-inflammatory diet (GrAID). In the process of treatment of oral and intestinal dysbiosis, probiotic therapy is beneficial in both diseases, specified as the Western diet. Non-conventional diets are not recommended.
Summary:
Diet therapy for inflammatory periodontal diseases and IBD requires extensive individualization; nevertheless, a universal principle is avoidance of highly processed food, and implementation of easily digestible meals based on natural, ecological products. Proper nutrition plays a crucial role in primary prevention of both diseases analyzed, whereas in secondary prevention, diet therapy is a valuable supplementation of pharmacotherapy.
REFERENCES (69)
1.
Goździewska M, Łyszczarz A, Kaczoruk M, Kolarzyk E. Relationship between periodontal diseases and non-specific inflammatory bowel diseases – an overview. Part I. Ann Agric Environ Med. 2024;31(1):1–7. https://doi.org/10.26444/aaem/....
2.
Jarosz M. Nowa piramida zdrowego żywienia i stylu życia dzieci i młodzieży. Żywienie Człowieka i Metabolizm. 2019;46(01):13–14.
3.
Jarosz M. Piramida Zdrowego Żywienia i Aktywności Fizycznej dla osób dorosłych. Narodowe Centrum Edukacji Żywieniowej https://ncez.pzh.gov.pl/abc-zy... (access: 2024.03.14).
4.
Jarosz M. Piramida Zdrowego Żywienia i Aktywności Fizycznej dla osób w wieku starszym. Narodowe Centrum Edukacji Żywieniowej https://ncez.pzh.gov.pl/senior... (access: 2024.03.14).
5.
Janion K, Walkiewicz K, Copija A, Nowakowska-Zajdel E. Praktyczne zalecenia żywieniowe w trakcie chemioterapii u chorych na nowotwory przewodu pokarmowego. Piel Pol. 2018;3(69):298–304. http://dx.doi.org/10.20883/pie....
6.
Pigneur B, Ruemmele FM. Nutritional interventions for the treatment of IBD: current evidence and controversies. Therap Adv Gastroenterol. 2019;25(12):1756284819890534. doi:10.1177/1756284819890534.
7.
Altun E, Walther C, Borof K, Petersen E, Lieske B, Kasapoudis D, et al. Association between Dietary Pattern and Periodontitis—A Cross-Sectional Study. Nutrients. 2021;13(11):4167. https://doi.org/10.3390/nu1311....
8.
Wright D, M, McKenna G, Nugent A, Winning L, et al. Association between diet and periodontitis: a cross-sectional study of 10,000 NHANES participants. AJCN. 2020;112(6):1485–1491.
9.
Li A, Chen Y, Schuller AA, van Der Sluis, et al. Dietary inflammatory potential is associated with poor periodontal health: A population-based study. J Clin Periodontol. 2021;48(7):907–918. doi:10.1111/jcpe.13472.
10.
Martinon P, Fraticelli L, Giboreau A, Dussart C, Bourgeois D, Carrouel F. Nutrition as a key modifiable factor for periodontitis and main chronic diseases. J Clin Med. 2021;10(2):197. https://doi.org/10.3390/jcm100....
11.
Jauhiainen LM, Ylöstalo PV, Knuuttila M, Männistö S, et al. Poor diet predicts periodontal disease development in 11-year follow-up study. Community Dent Oral Epidemiol. 2019;48(2):143–151. doi:10.1111/cdoe.12513.
12.
Santonocito S, Polizzi A, Palazzo G, Indelicato F, et al. Dietary factors affecting the prevalence and impact of periodontal disease. Clin Cosmet Investig Dent. 2021;13:283–292. doi:10.2147/CCIDE.S288137.
13.
Li W, Shang Q, Yang D, Peng J, et al. Abnormal Micronutrient Intake Is Associated with the Risk of Periodontitis: A Dose–response Association Study Based on NHANES 2009–2014. Nutrients. 2022;14(12):2466. doi:10.3390/nu14122466.
14.
Chatterjee D, Chatterjee A, Kalra D, Kapoor A, Vijay S, Jain S. Role of adjunct use of omega 3 fatty acids in periodontal therapy of periodontitis. A systematic review and meta-analysis. J Oral Biol Craniofac Res. 2022;12(1):55–62. https://doi.org/10.1016/j.jobc....
15.
Azuma MM, Cardoso CBM, da Silva CC, de Oliveira PHC, Jacinto RC, Andrada AC, et al. The use of omega-3 fatty acids in the treatment of oral diseases. Oral Dis. 2022;28(2):264–74. https://doi.org/10.1111/odi.13....
16.
Stando M, Piatek P, Namiecinska M, Lewkowicz P, Lewkowicz N. Omega-3 polyunsaturated fatty acids EPA and DHA as an adjunct to non-surgical treatment of periodontitis: a randomized clinical trial. Nutrients. 2020;12(9):2614. https://doi.org/10.3390/nu1209....
17.
Woelber JP, Gartner M, Breuninger L, Anderson A, Konig D, Hellwig E, et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J Clin Periodontol. 2019;46(4):481–90. https://doi.org/10.1111/jcpe.1....
18.
Kantorowicz M, Olszewska-Czyż I, Lipska W, Kolarzyk E, Chomyszyn-Gajewska M. Impact of dietary habits on the incidence of oral diseases. Dent Med Probl. 2022;59(4):547–554. doi:10.17219/dmp/134749.
19.
Casarin M, da Silveira TM, Bezerra B, Pirih F, et al. Association between different dietary patterns and eating disorders and periodontal diseases. Front Oral Health. 2023;22(4):1152031. doi:10.3389/froh.2023.1152031.
20.
Almoznino G, Gal N, Levin L, Mijiritsky E, et al. Diet Practices, Body Mass Index, and Oral Health-Related Quality of Life in Adults with Periodontitis – A Case – Control Study. Int J Environ Res Public Health. 2020;17(7):2340. https://doi.org/10.3390/ijerph....
21.
Wellapuli N, Ekanayake L. Association between chronic periodontitis and oral health-related quality of life in Sri Lankan adults. Int Dent J. 2016;66:337–343.
22.
Levine A, Rhodes JM, Lindsay JO, Abreu MT, et al. Dietary Guidance from the International Organization for the Study of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1381–1392. doi:10.1016/j.cgh.2020.01.046.
23.
Niezgódka Klósak A, Eder P. Dieta w nieswoistych chorobach zapalnych jelit. Omówienie wskazówek International Organization for the Study of Inflammatory Bowel Disease 2020. Med Prakt. 2021;11:58–65.
24.
Cox SR, Lindsay JO, Fromentin S, Stagg AJ, et al. Effects of Low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. 2020;158(1):176–188.e7. doi:10.1053/j.gastro.2019.09.024.
25.
Pedersen N, Ankersen DV, Felding M, Wachmann H, et al. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J Gastroenterol. 2017;14(23):3356–3366. doi:10.3748/wjg.v23.i18.3356.
26.
Schreiner P, Yilmaz B, Rossel JB, Franc Y, et al. Swiss IBD Cohort Study Group. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur Gastroenterol J. 2019;7(6):767–781. doi:10.1177/2050640619841249.
27.
Barnes EL, Nestor M, Onyewadume L, de Silva PS, Korzenik JR. DREAM Investigators. High dietary intake of specific fatty acids increases risk of flares in patients with ulcerative colitis in remission during treatment with aminosalicylates. Clin Gastroenterol Hepatol. 2017;15(9):1390–1396. doi:10.1016/j.cgh.2016.12.036.
28.
Barrett JS, Irving PM, Shepherd SJ, Muir JG, Gibson PR. Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment Pharmacol Ther. 2009;30:165–174. doi:10.1111/j.1365-2036.2009.04018.x.
29.
Owczarek D, Rodacki T, Domagała-Rodacka R, Cibor D, Mach T. Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol. 2016;21–22(3):895–905. doi:10.3748/wjg.v22.i3.895.
30.
Godala M, Gaszyńska E, Zatorski H, Małecka-Wojciesko E. Dietary interventions in inflammatory bowel disease. Nutrients. 2022;14(20):4261. doi:10.3390/nu14204261.
31.
Saha S, Patel N. What Should I Eat? Dietary Recommendations for patients with inflammatory bowel disease. Nutrients. 2023;15(4):896. doi:10.3390/nu15040896.
32.
Chicco F, Magrì S, Cingolani A, Paduano D, et al. Multidimensional impact of Mediterranean Diet on IBD patients. Inflamm. Bowel Dis. 2021;27(1):1–9. doi:10.1093/ibd/izaa097.
33.
Papada E, Amerikanou C, Forbes A, Kaliora AC. Adherence to Mediterranean diet in Crohn’s disease. Eur J Nutr. 2020;59:1115–1121. doi:10.1007/s00394-019-01972.
34.
Obih C, Wahbeh G, Lee D, Braly K, et al. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within and academic IBD center. Nutrition. 2016;32(4):418–425. doi:10.1016/j.nut.2015.08.025.
35.
Lewis JD, Sandler RS, Brotherton C, Brensinger C, et al. DINE-CD Study Group. A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease. Gastroenterology. 2021;161:837–852.e9. doi:10.1053/j.gastro.2021.05.047.
36.
Limketkai BN, Godoy-Brewer G, Parian AM, Noorian S, et al. Dietary Interventions for the Treatment of Inflammatory Bowel Diseases: An Updated Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21(10):2508–2525. doi:10.1016/j.cgh.2022.11.026.
37.
Campmans-Kuijpers MJE, Dijkstra G. Food and Food Groups in Inflammatory Bowel Disease (IBD): The Design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients. 2021;25;13(4):1067. doi:10.3390/nu13041067.
38.
Jaramillo AP, Abaza A, Sid Idris F, Anis H, et al. Diet as an Optional Treatment in Adults With Inflammatory Bowel Disease: A Systematic Review of the Literature. Cureus. 2023;18;15(7):e42057. doi:10.7759/cureus.42057.
39.
de Oliveira AM, Lourenço TGB, Colombo APV. Impact of systemic probiotics as adjuncts to subgingival instrumentation on the oral-gut microbiota associated with periodontitis: A randomized controlled clinical trial. J Periodontol. 2022;93(1):31–44. doi:10.1002/JPER.21-0078.
40.
Deandra FA, Ketherin K, Rachmasari R, Sulijaya B, Takahashi N. Probiotics and metabolites regulate the oral and gut microbiome composition as host modulation agents in periodontitis: A narrative review. Heliyon. 2023;3;9(2):e13475. doi:10.1016/j.heliyon.2023.e13475.
41.
Preidis GA, Weizman AV, Kashyap PC, Morgan RL. AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159(2):708–738.e4. doi:10.1053/j.gastro.2020.05.060.
42.
Su GL, Ko CW, Bercik P, Falck-Ytter Y, Sultan S, Weizman AV, Morgan RL. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159(2):697–705. doi:10.1053/j.gastro.2020.05.059.
43.
Lourenςo TGB, Spencer SJ, Alm EJ, Colombo APV. Defining the gut microbiota in individuals with periodontal diseases: an exploratory study. J Oral Microbiol. 2018;3;10(1):1487741. doi:10.1080/20002297.2018.1487741.
44.
World Health Organization. Ten threats to global health in 2019. https://www.who.int/news-room/... (access: 2024.03.18).
45.
Health Emergency Preparedness and Response Authority. HERA factsheet – HEALTH UNION: Identifying top 3 priority health threats https://health.ec.europa.eu/pu... (access: 2024.03.18).
46.
Mathewson ND, Jenq R, Mathew AV, Koenigsknecht M, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–513. doi:10.1038/ni.3400.
47.
Siddiqui R, Badran Z, Boghossian A, Alharbi AM, Alfahemi H, Khan NA. The increasing importance of the oral microbiome in periodontal health and disease. Future Sci OA. 2023;12;9(8):FSO856. doi:10.2144/fsoa-2023-0062.
48.
Ferrillo M, Giudice A, Migliario M, Renó F, et al. Oral–Gut Microbiota, Periodontal Diseases, and Arthritis: Literature Overview on the Role of Probiotics. Inter J Mol Sci. 2023;24(5):4626. https://doi.org/10.3390/ijms24....
49.
Minić I, Pejčić A, Bradić-Vasić M. Effect of the local probiotics in the therapy of periodontitis A randomized prospective study. Int J Dent Hyg. 2022;20(2):401–407. doi:10.1111/idh.12509.
50.
Kobayashi R, Kobayashi T, Sakai F, Hosoya T, Yamamoto M, Kurita-Ochiai T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Sci Rep. 2017;3;7(1):545. doi:10.1038/s41598-017-00623-9.
51.
Singh D, Khan MA, Siddique HR. Therapeutic implications of probiotics in microbiota dysbiosis: A special reference to the liver and oral cancers. Life Sci. 2021;15(285):120008. doi:10.1016/j.lfs.2021.120008.
52.
Choi Y, Park E, Kim S, Ha J, et al. Fermented milk with Lactobacillus curvatus SMFM2016-NK alleviates periodontal and gut inflammation, and alters oral and gut microbiota. J Dairy Sci. 2021;104(5):5197–5207. doi:10.3168/jds.2020-19625.
53.
Kong C, Yan X, LiuY, Huang L, et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. Sig Transduct Target Ther. 2021;6:154. https://doi.org/10.1038/s41392....
54.
Li S, Zhuge A, Wang K, Lv L, Bian X, Yang L, et al. Ketogenic diet aggravates colitis, impairs intestinal barrier and alters gut microbiota and metabolism in DSS-induced mice. Food Funct. 2021;19;12(20):10210–10225. doi:10.1039/d1fo02288a.
55.
Gubatan J, Kulkarni CV, Talamantes SM, Temby M, Fardeen T, Sinha SR. Dietary Exposures and Interventions in Inflammatory Bowel Disease: Current Evidence and Emerging Concepts. Nutrients. 2023;15(3):579. https://doi.org/10.3390/nu1503....
56.
Paoli A, Moro T, Bosco G, Bianco A, Grimaldi KA, Camporesi E, Mangar D. Effects of n-3 Polyunsaturated Fatty Acids (ω-3) Supplementation on Some Cardiovascular Risk Factors with a Ketogenic Mediterranean Diet. Marine Drugs. 2015;13(2):996–1009. https://doi.org/10.3390/md1302....
57.
Rondanelli M, Perna S, Ilyas Z, Peroni G, Bazire P, Sajuox I, et al. Effect of very low-calorie ketogenic diet in combination with omega-3 on inflammation, satiety hormones, body composition, and metabolic markers. A pilot study in class I obese subjects. Endocrine. 2022;75(1):129–136. doi:10.1007/s12020-021-02860-5.
58.
Ho SM, Lewis JD, Mayer EA, Plevy SE, et al. Challenges in IBD Research: Environmental Triggers. Inflamm. Bowel Dis. 2019;16;25(Suppl 2):S13-S23. doi:10.1093/ibd/izz076.
59.
Rizzello F, Spisni E, Giovanardi E, Imbesi V, et al. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients. 2019;8;11(5):1033. doi:10.3390/nu11051033.
60.
Krela-Kaźmierczak I, Zakerska-Banaszak O, Skrzypczak-Zielińska M, Łykowska-Szuber L, A et al. Where Do We Stand in the Behavioral Pathogenesis of Inflammatory Bowel Disease? The Western Dietary Pattern and Microbiota-A Narrative Review. Nutrients. 2022;17;14(12):2520. doi:10.3390/nu14122520.
61.
Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019 1;2019:7247238. doi:10.1155/2019/7247238.
62.
Dolan KT, Chang EB. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol Nutr Food Res. 2017;61(1):10.1002/mnfr.201600129. doi:10.1002/mnfr.201600129.
63.
Schwärzler J, Mayr L, Vich Vila A, Grabherr F, et al. PUFA-Induced Metabolic Enteritis as a Fuel for Crohn’s Disease. Gastroenterology. 2022;162(6):1690–1704. doi:10.1053/j.gastro.2022.01.004.
64.
Rinninella E, Raoul P, Cintoni M, Franceschi F, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;10;7(1):14. doi:10.3390/microorganisms7010014.
65.
Niewiadomski O, Studd C, Wilson J, Williams J, et al. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern Med J. 2016;46:669–676. doi:10.1111/imj.13094.
66.
CaoY, Liu H, Qin N, Ren X, et al. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci Technol. 2020;99:295–310. https://doi.org/10.1016/j.tifs....
67.
Xu Y, Luo J, Gao Y, Tao Y, Xu J, Yao T, Chen Y. Causal effects between inflammatory bowel disease and oral diseases based on Oral-GUT Axis: a Mendelian randomization study. Nutrients 2023;15(20):4445 https://doi.org/10.3390/nu1520....
68.
Byrd KM, Gulati AS. The “Gum-Gut” Axis in Inflammatory Bowel Diseases: A Hypothesis-Driven Review of Associations and Advances. Front Immunol. 2021 Feb 19;12:620124. doi:10.3389/fimmu.2021.620124. PMID:33679761; PMCID:PMC7933581.
69.
Virk MS, Virk MA, He Y, Tufail T, et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients. 2024;16(4):546. https://doi.org/10.3390/nu1604....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.