Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
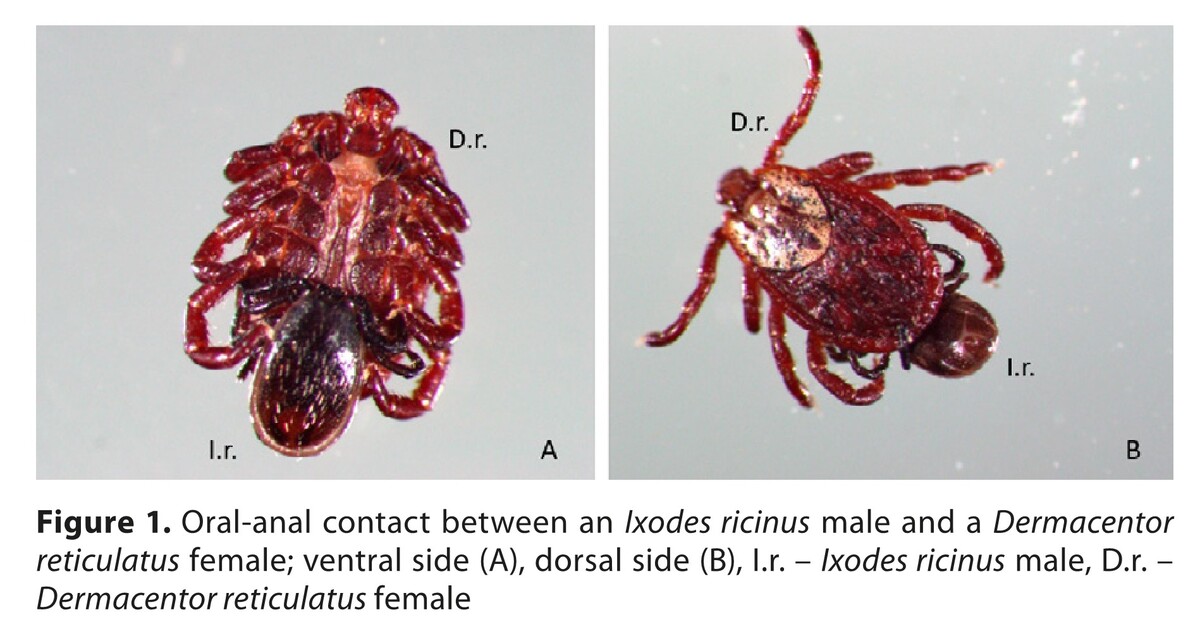
Prevalence of pathogens in sympatric Ixodes ricinus and Dermacentor reticulatus ticks in Eastern Poland and their potential impact on oral-anal contacts between ticks
1
Department of Biology and Parasitology, Medical University, Lublin, Poland
2
Department of Parasitology, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia, Katowice, Poland
3
Faculty of Medical Sciences in Zabrze, Department of Medical and Molecular Biology, Medical University of Silesia, Katowice, Poland
Corresponding author
Alicja Buczek
Medical University, Department of Biology and Parasitology, ul. Radziwiłłowska 11, 20-080, Lublin, Poland
Medical University, Department of Biology and Parasitology, ul. Radziwiłłowska 11, 20-080, Lublin, Poland
Ann Agric Environ Med. 2023;30(2):259-265
KEYWORDS
Ixodes ricinusDermacentor reticulatusco-infectionstick behaviouroral-anal contact between tickstick-borne pathogensvector manipulation
TOPICS
ABSTRACT
Introduction and objective:
Little is known about interspecific contacts between ticks. Therefore, this study focused on the investigation of factors that may influence interspecific contacts between Ixodes ricinus and Dermacentor reticulatus ticks.
Material and methods:
Ixodes ricinus males and D. reticulatus females involved in oral-anal contacts (group I) and questing specimens with no such behaviour (group II) collected in eastern Poland were examined using molecular techniques to detect Borrelia burgdorferi s.l. (Bb), Rickettsia spp. (Rs), Anaplasma phagocytophilum, Babesia microti, and Toxoplasma gondii.
Results:
An extremely high infection rate of Bb and Rs was determined in I. ricinus males (in groups I: 100% and 46.15% and group II: 90% and 40%, respectively) and D. reticulatus females (in group I: 84.61% and 61.53% and in group II: 90% and 20%, respectively). The prevalence of other pathogens in these ticks was substantially lower. Co-infection with pathogens was detected in approximately 53% of ticks.
Conclusions:
The study suggests that tick-borne pathogens may have influenced the sexual behaviour of their vectors. The oral-anal contacts between I. ricinus and D. reticulatus ticks are probably stimulated by Bb and/or Rs. The presence of five pathogens and numerous co-infections in the analysed ticks indicates a risk of various human infectious diseases in the study region. Further studies are required to clarify the implications of oral-anal interspecific tick interactions.
Little is known about interspecific contacts between ticks. Therefore, this study focused on the investigation of factors that may influence interspecific contacts between Ixodes ricinus and Dermacentor reticulatus ticks.
Material and methods:
Ixodes ricinus males and D. reticulatus females involved in oral-anal contacts (group I) and questing specimens with no such behaviour (group II) collected in eastern Poland were examined using molecular techniques to detect Borrelia burgdorferi s.l. (Bb), Rickettsia spp. (Rs), Anaplasma phagocytophilum, Babesia microti, and Toxoplasma gondii.
Results:
An extremely high infection rate of Bb and Rs was determined in I. ricinus males (in groups I: 100% and 46.15% and group II: 90% and 40%, respectively) and D. reticulatus females (in group I: 84.61% and 61.53% and in group II: 90% and 20%, respectively). The prevalence of other pathogens in these ticks was substantially lower. Co-infection with pathogens was detected in approximately 53% of ticks.
Conclusions:
The study suggests that tick-borne pathogens may have influenced the sexual behaviour of their vectors. The oral-anal contacts between I. ricinus and D. reticulatus ticks are probably stimulated by Bb and/or Rs. The presence of five pathogens and numerous co-infections in the analysed ticks indicates a risk of various human infectious diseases in the study region. Further studies are required to clarify the implications of oral-anal interspecific tick interactions.
ACKNOWLEDGEMENTS
The authors are grateful to Katarzyna Bartosik and Daniel
Brzozowski for finding the locality where the ticks were
collected for our study and Aneta Woźniak for collecting
the ticks in the habitat.
FUNDING
Publication financed by the Medical University of Lublin, Poland (Grant No. DS507).
REFERENCES (67)
1.
Bartosik K, Sitarz M, Szymańska J, Buczek A. Tick bites on humans in the agricultural and recreational areas in south-eastern Poland. Ann Agric Environ Med. 2011;18(1):151–157.
2.
Kalmár Z, Dumitrache MO, D’Amico G, et al. Multiple Tick-Borne Pathogens in Ixodes ricinus Ticks Collected from Humans in Romania. Pathogens. 2020;9(5):390. Published 2020 May 19. doi:10.3390/pathogens9050390.
3.
Pawełczyk A, Bednarska M, Hamera A, et al. Long-term study of Borrelia and Babesia prevalence and co-infection in Ixodes ricinus and Dermacentor recticulatus ticks removed from humans in Poland, 2016–2019. Parasit Vectors. 2021;14(1):348. Published 2021 Jul 1. doi:10.1186/s13071-021-04849-5.
4.
Földvári G, Rigó K, Lakos A. Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagn Microbiol Infect Dis. 2013;76(3):387–389. doi:10.1016/j.diagmicrobio.2013.03.005.
5.
Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013;6:1. Published 2013 Jan 2. doi:10.1186/1756-3305-6-1.
6.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016;9(1):314. Published 2016 Jun 1. doi:10.1186/s13071-016-1599-x.
7.
Pangrácová L, Derdáková M, Pekárik L, et al. Ixodes ricinus abundance and its infection with the tick-borne pathogens in urban and suburban areas of Eastern Slovakia. Parasit Vectors. 2013;6(1):238. Published 2013 Aug 16. doi:10.1186/1756-3305-6-238.
8.
Kohn M, Krücken J, McKay-Demeler J, Pachnicke S, Krieger K, von Samson-Himmelstjerna G. Dermacentor reticulatus in Berlin/Brandenburg (Germany): Activity patterns and associated pathogens. Ticks Tick Borne Dis. 2019;10(1):191–206. doi:10.1016/j.ttbdis.2018.10.003.
9.
Mancini F, Vescio MF, Toma L, et al. Detection of tick-borne pathogens in ticks collected in the suburban area of Monte Romano, Lazio Region, Central Italy. Ann Ist Super Sanita. 2019;55(2):143–150. doi:10.4415/ANN_19_02_06.
10.
Snegiriovaite J, Radzijevskaja J, Paulauskas A. A brief review: the prevalence of tick-borne pathogens in urban and suburban areas. Biologija. 2020;66(4):242–255.
11.
Borşan SD, Toma-Naic A, Péter Á, Sándor AD, Pe?tean C, Mihalca AD. Impact of abiotic factors, habitat type and urban wildlife on the ecology of hard ticks (Acari: Ixodidae) in urban and peri-urban habitats. Parasit Vectors. 2020;13(1):476. Published 2020 Sep 18. doi:10.1186/s13071-020-04352-3.
12.
Grochowska A, Dunaj-Małyszko J, Pancewicz S, et al. Prevalence of Tick-Borne Pathogens in Questing Ixodes ricinus and Dermacentor reticulatus Ticks Collected from Recreational Areas in Northeastern Poland with Analysis of Environmental Factors. Pathogens. 2022;11(4):468. Published 2022 Apr 14. doi:10.3390/pathogens11040468.
13.
Carroll JF, Mills GD Jr, Schmidtmann ET. Field and laboratory responses of adult Ixodes scapularis (Acari: Ixodidae) to kairomones produced by white-tailed deer. J Med Entomol. 1996;33(4):640–644. doi:10.1093/jmedent/33.4.640.
14.
Wanzala W, Sika NFK, Gule S, et al. Attractive and repellent host odours guide ticks to their respective feeding sites. Chemoecology. 2004;14:29–232. https://doi.org/10.1007/s00049....
15.
Crooks E, Randolph SE. Walking by Ixodes ricinus ticks: intrinsic and extrinsic factors determine the attraction of moisture or host odour. J Exp Biol. 2006;209(Pt 11):2138–2142. doi:10.1242/jeb.02238.
16.
Alekseev AN. Tick pathogen interactions: behavior of infected and uninfected ticks (Ixodidae). In: Mitchell R, Horn DJ, Needham GR, Welbourn W, editors. Acarology. Columbus: Biological Survey; 1996. p. 113–15.
17.
Alekseev AN, Dubinina HV. Abiotic parameters and diel and seasonal activity of Borrelia-infected and uninfected Ixodes persulcatus (Acarina: Ixodidae). J Med Entomol. 2000;37(1):9–15. doi:10.1603/0022-2585-37.1.9.
18.
Lefcort H, Durden LA. The effect of infection with Lyme disease spirochetes (Borrelia burgdorferi) on the phototaxis, activity, and questing height of the tick vector Ixodes scapularis. Parasitology. 1996;113 (Pt 2):97–103. doi:10.1017/s0031182000066336.
19.
Belova OA, Burenkova LA, Karganova GG. Different tick-borne encephalitis virus (TBEV) prevalences in unfed versus partially engorged ixodid ticks--evidence of virus replication and changes in tick behavior. Ticks Tick Borne Dis. 2012;3(4):240–246. doi:10.1016/j.ttbdis.2012.05.005.
20.
Herrmann C, Gern L. Do the level of energy reserves, hydration status and Borrelia infection influence walking by Ixodes ricinus (Acari: Ixodidae) ticks? Parasitology. 2012;139(3):330–337. doi:10.1017/S0031182011002095.
21.
Herrmann C, Gern L. Search for blood or water is influenced by Borrelia burgdorferi in Ixodes ricinus. Parasit Vectors. 2015;8:6. Published 2015 Jan 6. doi:10.1186/s13071-014-0526-2.
22.
Benelli G. Pathogens Manipulating Tick Behavior-Through a Glass, Darkly. Pathogens. 2020;9(8):664. Published 2020 Aug 17. doi:10.3390/pathogens9080664.
23.
Javed N, Bhatti A, Paradkar PN. Advances in Understanding Vector Behavioural Traits after Infection. Pathogens. 2021;10(11):1376. Published 2021 Oct 24. doi:10.3390/pathogens10111376.
24.
Buczek A, Bartosik K, Buczek W, et al. A unique phenomenon of oral-anal contact between ticks observed in two tick species Ixodes ricinus and Dermacentor reticulatus. Ann Agric Environ Med. 2018;25(4):686–689. doi:10.26444/aaem/99054 https://doi.org/10.26444/aaem/....
25.
Guy EC, Stanek G. Detection of Borrelia burgdorferi in patients with Lyme disease by the polymerase chain reaction. J Clin Pathol. 1991;44(7):610–611. doi:10.1136/jcp.44.7.610.
26.
Wodecka B, Rymaszewska A, Sawczuk M, Skotarczak B. Detectability of tick-borne agents DNA in the blood of dogs, undergoing treatment for borreliosis. Ann Agric Environ Med. 2009;16(1):9–14.
27.
Massung RF, Slater K, Owens JH, et al. Nested PCR assay for detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36(4):1090–1095. doi:10.1128/JCM.36.4.1090-1095.1998.
28.
Persing DH, Mathiesen D, Marshall WF, et al. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30(8):2097–2103. doi:10.1128/jcm.30.8.2097-2103.1992.
29.
Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173(5):1576–1589. doi:10.1128/jb.173.5.1576-1589.1991.
30.
Sroka J, Wójcik-Fatla A, Zwoliński J, Zajac V, Sawczuk M, Dutkiewicz J. Preliminary study on the occurrence of Toxoplasma gondii in Ixodes ricinus ticks from north-western Poland with the use of PCR. Ann Agric Environ Med. 2008;15(2):333–338.
31.
Bartosik K, Buczek A, Borzęcki A, Kulina D. Study of the non-parasitic stage in Ixodes ricinus after co-feeding with Dermacentor reticulatus in three infestations. Ann Agric Environ Med. 2017;24(1):90–95. doi:10.5604/12321966.1234005.
32.
Buczek A, Bartosik K, Zając Z, Stanko M. Host-feeding behaviour of Dermacentor reticulatus and Dermacentor marginatus in mono-specific and inter-specific infestations. Parasit Vectors. 2015;8:470. Published 2015 Sep 17. doi:10.1186/s13071-015-1078-9.
33.
Kagemann J, Clay K. Effects of infection by Arsenophonus and Rickettsia bacteria on the locomotive ability of the ticks Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis. J Med Entomol. 2013;50(1):155–162. doi:10.1603/me12086.
34.
Richardson EA, Taylor CE, Jabot B, Martin E, Keiser CN. The effects of habitat type and pathogen infection on tick host-seeking behaviour. Parasitology. 2022;149(1):59–64. doi:10.1017/S0031182021001554.
35.
Hu R, Hyland KE, Markowski D. Effects of Babesia microti infection on feeding pattern, engorged body weight, and molting rate of immature Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 1997;34(5):559–564. doi:10.1093/jmedent/34.5.559.
36.
Villar M, López V, Ayllón N, et al. The intracellular bacterium Anaplasma phagocytophilum selectively manipulates the levels of vertebrate host proteins in the tick vector Ixodes scapularis. Parasit Vectors. 2016;9(1):467. Published 2016 Aug 25. doi:10.1186/s13071-016-1747-3.
37.
Reye AL, Stegniy V, Mishaeva NP, et al. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks fromdifferent geographical locations in Belarus. PLoS One. 2013;8(1):e54476. doi:10.1371/journal.pone.0054476.
38.
Richter D, Kohn C, Matuschka FR. Absence of Borrelia spp., Candidatus Neoehrlichia mikurensis, and Anaplasma phagocytophilum in questing adult Dermacentor reticulatus ticks. Parasitol Res. 2013;112(1):107–111. doi:10.1007/s00436-012-3110-8.
39.
Rudolf I, Hubálek Z. Effect of the salivary gland and midgut extracts from Ixodes ricinus and Dermacentor reticulatus (Acari: Ixodidae) on the growth of Borrelia garinii in vitro. Folia Parasitol (Praha). 2003;50(2):159–160.
40.
Wójcik-Fatla A, Szymańska J, Wdowiak L, Buczek A, Dutkiewicz J. Coincidence of three pathogens (Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti) in Ixodes ricinus ticks in the Lublin macroregion. Ann Agric Environ Med. 2009;16(1):151–158.
41.
Wójcik-Fatla A, Zając V, Sawczyn A, Sroka J, Cisak E, Dutkiewicz J. Infections and mixed infections with the selected species of Borrelia burgdorferi sensu lato complex in Ixodes ricinus ticks collected in eastern Poland: a significant increase in the course of 5 years. Exp Appl Acarol. 2016;68(2):197–212. doi:10.1007/s10493-015-9990-4.
42.
Zając V, Wójcik-Fatla A, Sawczyn A, et al. Prevalence of infections and co-infections with 6 pathogens in Dermacentor reticulatus ticks collected in eastern Poland. Ann Agric Environ Med. 2017;24(1):26–32. doi:10.5604/12321966.1233893.
43.
Pańczuk A, Tokarska-Rodak M, Teodorowicz P, Pawłowicz-Sosnowska E. Tick-borne pathogens in Dermacentor reticulatus collected from dogs in eastern Poland. Exp Appl Acarol. 2022;86(3):419–429. doi:10.1007/s10493-022-00700-3.
44.
Roczeń-Karczmarz M, Dudko P, Demkowska-Kutrzepa M, et al. Comparison of the occurrence of tick-borne diseases in ticks collected from vegetation and animals in the same area. Med Wet. 2018;74(8):484–488.
45.
Kowalec M, Szewczyk T, Welc-Falęciak R, Siński E, Karbowiak G, Bajer A. Ticks and the city – are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit Vectors. 2017;10(1):573. Published 2017 Nov 21. doi:10.1186/s13071-017-2391-2.
46.
Mierzejewska EJ, Pawełczyk A, Radkowski M, Welc-Falęciak R, Bajer A. Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit Vectors. 2015;8:490. Published 2015 Sep 24. doi:10.1186/s13071-015-1099-4.
47.
Dunaj J, Trzeszczkowski A, Moniuszko-Malinowska A, Rutkowski K, Pancewicz S. Assessment of tick-borne pathogens presence in Dermacentor reticulatus ticks in north-eastern Poland. Adv Med Sci. 2021;66(1):113–118. doi:10.1016/j.advms.2021.01.002.
48.
Kubiak K, Dziekońska-Rynko J, Szymańska H, Kubiak D, Dmitryjuk M, Dzika E. Questing Ixodes ricinus ticks (Acari, Ixodidae) as a vector of Borrelia burgdorferi sensu lato and Borrelia miyamotoi in an urban area of north-eastern Poland. Exp Appl Acarol. 2019;78(1):113–126. doi:10.1007/s10493-019-00379-z.
49.
Michalski MM, Kubiak K, Szczotko M, Dmitryjuk M. Tick-Borne Pathogens in Ticks Collected from Wild Ungulates in North-Eastern Poland. Pathogens. 2021;10(5):587. Published 2021 May 11. doi:10.3390/pathogens10050587.
50.
Buczek A, Ciura D, Bartosik K, Zając Z, Kulisz J. Threat of attacks of Ixodes ricinus ticks (Ixodida: Ixodidae) and Lyme borreliosis within urban heat islands in south-western Poland. Parasit Vectors. 2014;7:562. Published 2014 Dec 11. doi:10.1186/s13071-014-0562-y.
51.
Król N, Kiewra D, Szymanowski M, Lonc E. The role of domestic dogs and cats in the zoonotic cycles of ticks and pathogens. Preliminary studies in the Wrocław Agglomeration (SW Poland). Vet Parasitol. 2015;214(1–2):208–212. doi:10.1016/j.vetpar.2015.09.028.
52.
Asman M, Witecka J, Solarz K, Zwonik A, Szilman P. Occurrence of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in Ixodes ricinus ticks collected from selected areas of Opolskie Province in south-west Poland. Ann Agric Environ Med. 2019;26(4):544–547. doi:10.26444/aaem/110214.
53.
Kocoń A, Asman M, Nowak-Chmura M, Witecka J, Rączka G. Exposure of domestic dogs and cats to ticks (Acari: Ixodida) and selected tick-borne diseases in urban and recreational areas in southern Poland. Sci Rep. 2022;12(1):7851. Published 2022 May 12. doi:10.1038/s41598-022-11973-4.
54.
Sprong H, Fonville M, Docters van Leeuwen A, et al. Detection of pathogens in Dermacentor reticulatus in northwestern Europe: evaluation of a high-throughput array. Heliyon. 2019;5(2):e01270. Published 2019 Feb 28. doi:10.1016/j.heliyon.2019.e01270.
55.
Capligina V, Seleznova M, Akopjana S, et al. Large-scale countrywide screening for tick-borne pathogens in field-collected ticks in Latvia during 2017–2019. Parasit Vectors. 2020;13(1):351. Published 2020 Jul 14. doi:10.1186/s13071-020-04219-7.
56.
Răileanu C, Tauchmann O, Silaghi C. Sympatric occurrence of Ixodes ricinus with Dermacentor reticulatus and Haemaphysalis concinna and the associated tick-borne pathogens near the German Baltic coast. Parasit Vectors. 2022;15(1):65. Published 2022 Feb 22. doi:10.1186/s13071-022-05173-2.
57.
Ben I, Lozynskyi I. Prevalence of Anaplasma phagocytophilum in Ixodes ricinus and Dermacentor reticulatus and Coinfection with Borrelia burgdorferi and Tick-Borne Encephalitis Virus in Western Ukraine. Vector Borne Zoonotic Dis. 2019;19(11):793–801. doi:10.1089/vbz.2019.2450.
58.
Sormunen JJ, Andersson T, Aspi J, et al. Monitoring of ticks and tick-borne pathogens through a nationwide research station network in Finland. Ticks Tick Borne Dis. 2020;11(5):101449. doi:10.1016/j.ttbdis.2020.101449.
59.
Welc-Falęciak R, Kowalec M, Karbowiak G, Bajer A, Behnke JM, Siński E. Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural forested areas of Poland. Parasit Vectors. 2014;7:121. Published 2014 Mar 24. doi:10.1186/1756-3305-7-121.
60.
Kiewra D, Czułowska A. Prevalence of Rickettsia spp. in questing Ixodes ricinus (L. 1758) and Dermacentor reticulatus (Fabr. 1794) ticks in the Wroclaw agglomeration, south-west Poland. Preliminary study. Ann Parasitol. 2016;62:185.
61.
Chmielewski T, Podsiadly E, Karbowiak G, Tylewska-Wierzbanowska S. Rickettsia spp. in ticks, Poland. Emerg Infect Dis. 2009;15(3):486–488. doi:10.3201/eid1503.080711.
62.
Dwużnik-Szarek D, Mierzejewska EJ, Kiewra D, Czułowska A, Robak A, Bajer A. Update on prevalence of Babesia canis and Rickettsia spp. in adult and juvenile Dermacentor reticulatus ticks in the area of Poland (2016–2018). Sci Rep. 2022;12(1):5755. Published 2022 Apr 6. doi:10.1038/s41598-022-09419-y.
63.
Moutailler S, Valiente Moro C, Vaumourin E, et al. Co-infection of Ticks: The Rule Rather Than the Exception. PLoS Negl Trop Dis. 2016;10(3):e0004539. Published 2016 Mar 17. doi:10.1371/journal.pntd.0004539.
64.
Vyrostekova, V. [Transstadial transmission of Francisella tularensis in the tick, Ixodes ricinus, infected during the larval stage]. Cesk Epidemiol Mikrobiol. Imunol. 1993:42:71–75.
65.
Körner S, Makert GR, Mertens-Scholz K, et al. Uptake and fecal excretion of Coxiella burnetii by Ixodes ricinus and Dermacentor marginatus ticks. Parasit Vectors. 2020;13(1):75. Published 2020 Feb 14. doi:10.1186/s13071-020-3956-z.
66.
Wechtaisong W, Bonnet SI, Lien YY, Chuang ST, Tsai YL. Transmission of Bartonella henselae within Rhipicephalus sanguineus: Data on the Potential Vector Role of the Tick. PLoS Negl Trop Dis. 2020;14(10):e0008664. Published 2020 Oct 1. doi:10.1371/journal.pntd.0008664.
67.
Patton TG, Dietrich G, Gilmore RD Jr. Detection of Borrelia burgdorferi DNA in tick feces provides evidence for organism shedding during vector feeding. Vector Borne Zoonotic Dis. 2011;11(3):197–200. doi:10.1089/vbz.2010.0149.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.