Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
BRIEF COMMUNICATION
Preliminary analysis of oral and gut microbiome of an elderly patient with late-diagnosed phenylketonuria
1
Department of Biotechnology, Microbiology and Human Nutrition, University of Life Sciences, Lublin, Poland
Corresponding author
Elwira Komoń-Janczara
Department of Biotechnology, Microbiology and Human Nutrition, University of Life Sciences in Lublin, 8 Skromna St., 20-704, Lublin, Poland
Department of Biotechnology, Microbiology and Human Nutrition, University of Life Sciences in Lublin, 8 Skromna St., 20-704, Lublin, Poland
Ann Agric Environ Med. 2023;30(4):779-782
KEYWORDS
TOPICS
ABSTRACT
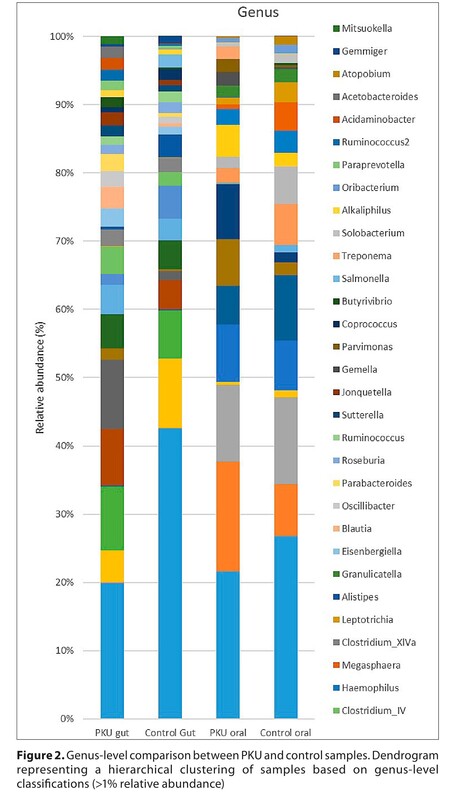
Phenylketonuria (PKU) is a metabolic and genetic disorder caused by a phenylalanine hydroxylase (PAH) gene deficiency that raises Phe levels in organs. Dietary therapy involves an elimination diet and Phe-free items, which may alter microbiota. The study examined the oral and intestinal microbiomes of a 63-year-old PKU patient and a control man, living in rural areas. iSeq100 (Illumina) sequenced the stool and oral 16S rRNA gene V3-V4 region. PKU guts had more Firmicutes and fewer Bacteroidetes than control. Clostridia predominated in PKU, while Bacteroidia dominated in control. Oral Bacteroidetes. Firmicutes, Proteobacteria, and Fusobacteria phyla were similar in both men. The microbiome may differ from those fed a Phe-free diet from birth due to late diagnosis and treatment of PKU. Due to the age of the 63-year-old patient’s and late therapy, the results differ from earlier studies. No study has compared an older PKU patient’s gut and oral microbiomes.
FUNDING
National Science Centre, project number: 2022/06/X/NZ9/00519 (ID 555260) and Polish Ministry of Education and Science/ University of Life Sciences: project number VKT/MN-7/TŻ/21.
REFERENCES (24)
1.
Giżewska M, MacDonald A, Bélanger-Quintana A, et al. Diagnostic and management practices for phenylketonuria in 19 countries of the South and Eastern European Region: survey results. Eur J Pediatr. 2016;175(2):261–272. doi:10.1007/S00431-015-2622-5/FIGURES/4.
3.
Montanari C, Ceccarani C, Corsello A, et al. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients. 2022;14(9):1883. doi:10.3390/NU14091883.
4.
MacDonald A, van Wegberg AMJ, Ahring K, et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J Rare Dis. 2020;15(1):1–21. doi:10.1186/S13023-020-01391-Y/TABLES/15.
5.
Pinheiro de Oliveira F, Mendes RH, Dobbler PT, et al. Phenylketonuria and Gut Microbiota: A Controlled Study Based on Next-Generation Sequencing. PLoS One. 2016;11(6):e0157513. doi:10.1371/JOURNAL.PONE.0157513.
6.
Ney DM, Murali SG, Stroup BM, et al. Metabolomic changes demonstrate reduced bioavailability of tyrosine and altered metabolism of tryptophan via the kynurenine pathway with ingestion of medical foods in phenylketonuria. Mol Genet Metab. 2017;121(2):96–103. doi:10.1016/J.YMGME.2017.04.003.
7.
Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell. 2018;9(5):488. doi:10.1007/S13238-018-0548-1.
8.
Kirby TO, Ochoa-Reparaz J, Roullet JB, Gibson KM. Dysbiosis of the intestinal microbiome as a component of pathophysiology in the inborn errors of metabolism. Mol Genet Metab. 2021;132(1):1–10. doi:10.1016/J.YMGME.2020.12.289.
9.
Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health. 2004;16(1):41–45. doi:10.1515/IJAMH.2004.16.1.41.
10.
MacLeod EL, Ney DM. Nutritional Management of Phenylketonuria. Annales Nestlé. 2010;68(2):58. doi:10.1159/000312813.
11.
Soltanizadeh N, Mirmoghtadaie L. Strategies Used in Production of Phenylalanine-Free Foods for PKU Management. Compr Rev Food Sci Food Saf. 2014;13(3):287–299. doi:10.1111/1541-4337.12057.
12.
Firman SJ, Ramachandran R, Whelan K, Witard OC, O’Keeffe M. Protein status in phenylketonuria: A scoping review. Clin Nutrition. 2022;41(4):894–922. doi:10.1016/J.CLNU.2022.02.010.
13.
Sailer M, Elizondo G, Martin J, Harding CO, Gillingham MB. Nutrient intake. body composition. and blood phenylalanine control in children with phenylketonuria compared to healthy controls. Mol Genet Metab Rep. 2020;23:100599. doi:10.1016/J.YMGMR.2020.100599.
14.
Zhao J, Zhang X, Liu H, Brown MA, Qiao S. Dietary Protein and Gut Microbiota Composition and Function. Curr Protein Pept Sci. 2018;20(2):145–154. doi:10.2174/1389203719666180514145437.
15.
Mancilla VJ, Mann AE, Zhang Y, Allen MS. The Adult Phenylketonuria (PKU) Gut Microbiome. Microorganisms. 2021;9(3):1–13. doi:10.3390/MICROORGANISMS9030530.
16.
Bassanini G, Ceccarani C, Borgo F, et al. Phenylketonuria Diet Promotes Shifts in Firmicutes Populations. Front Cell Infect Microbiol. 2019;9(MAR):101. doi:10.3389/FCIMB.2019.00101.
17.
Su Y, Shadike Q, Wang M, et al. A low abundance of genus Bacteroides in gut microbiota is negatively correlated with blood phenylalanine levels in Uygur patients with phenylketonuria. Transl Pediatr. 2021;10(10):2521–2532. doi:10.21037/TP-21-426.
18.
Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi:10.1038/nature11053.
19.
Verduci E, Moretti F, Bassanini G, et al. Phenylketonuric diet negatively impacts on butyrate production. Nutrit Metab Cardiov Dis. 2018;28(4):385–392. doi:10.1016/J.NUMECD.2018.01.004.
20.
Timmer C, Davids M, Nieuwdorp M, et al. Differences in faecal microbiome composition between adult patients with UCD and PKU and healthy control subjects. Mol Genet Metab Rep. 2021;29:100794. doi:10.1016/J.YMGMR.2021.100794.
21.
Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8(4):573. doi:10.3390/MICROORGANISMS8040573.
22.
Ashe K, Kelso W, Farrand S, et al. Psychiatric and Cognitive Aspects of Phenylketonuria: The Limitations of Diet and Promise of New Treatments. Front Psychiatry. 2019;10:561. doi:10.3389/FPSYT.2019.00561.
23.
van der Goot E, Vink SN, van Vliet D, van Spronsen FJ, Falcao Salles J, van der Zee EA. Gut-Microbiome Composition in Response to Phenylketonuria Depends on Dietary Phenylalanine in BTBR Pahenu2 Mice. Front Nutr. 2022;8:735366. doi:10.3389/fnut.2021.735366.
24.
Tufekcioglu Z, Cakar A, Bilgic B, Hanagasi H, Gurvit H, Emre M. Adult-onset phenylketonuria with rapidly progressive dementia and parkinsonism. Neurocase. 2016;22(3):273–275. doi:10.1080/13554794.2016.1142567.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.