Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Mycobiota of berry fruits – levels of filamentous fungi and mycotoxins, composition of fungi, and analysis of potential health risk for consumers
1
Department of Health Biohazards and Parasitology, Institute of Rural Health, Lublin, Poland
Corresponding author
Teresa Kłapeć
Corresponding author. Department of Health Biohazards and Parasitology, Institute of Rural Health, Jaczewskiego 2,, 20-090, Lublin, Poland
Corresponding author. Department of Health Biohazards and Parasitology, Institute of Rural Health, Jaczewskiego 2,, 20-090, Lublin, Poland
Ann Agric Environ Med. 2022;29(1):28-37
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
The aim of the study was to determine the presence, concentration and generic composition of filamentous fungi. Selected mycotoxins were also determined: total aflatoxins and deoxynivalenol.
Material and methods:
In 2017–2018, 40 samples of strawberry fruits and 40 samples of red raspberry fruits were collected. In 2019–2020, 37 samples of fresh strawberry fruits and 41 samples of fresh red raspberry fruits were collected on conventional farms located in eastern Poland and were subjected to mycological examination. The concentration and species composition of filamentous fungi were determined by the method of plate dilutions on malt agar. The isolated strains were identified using macroscopic and microscopic methods. Samples were also analysed for the presence of aflatoxin B1, total aflatoxin and deoxynivalenol using ELISA tests.
Results:
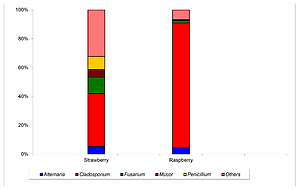
The median concentrations of fungi were moderate or low: 3.079 and 3.491 log10 CFU g-1 for strawberries and raspberries, respectively. Fungi of the genus Cladosporium prevailed in the mycobiota of berry fruits, accounting for 84.3% of total isolates in raspberries and 34.8% in strawberries. The occurrence of total aflatoxins was detected in the majority of tested samples (73.2% in raspberries and 70.3% in strawberries) but never exceeded the level of 4.0 µg kg-1 assumed as safe. Deoxynivalenol has been detected only in raspberries with the prevalence of 58.5%. Its median concentration was 242.0 µg kg-1 and in 7 out of 41 samples (17.0%) exceeded the level of 750.0 µg kg-1, assumed as safe.
Conclusions:
Filamentous fungi and mycotoxins occurred in the examined berries at levels that mostly do not represent a health risk for immunocompetent people, but might pose such risk for immuno-compromised and/or atopic consumers.
The aim of the study was to determine the presence, concentration and generic composition of filamentous fungi. Selected mycotoxins were also determined: total aflatoxins and deoxynivalenol.
Material and methods:
In 2017–2018, 40 samples of strawberry fruits and 40 samples of red raspberry fruits were collected. In 2019–2020, 37 samples of fresh strawberry fruits and 41 samples of fresh red raspberry fruits were collected on conventional farms located in eastern Poland and were subjected to mycological examination. The concentration and species composition of filamentous fungi were determined by the method of plate dilutions on malt agar. The isolated strains were identified using macroscopic and microscopic methods. Samples were also analysed for the presence of aflatoxin B1, total aflatoxin and deoxynivalenol using ELISA tests.
Results:
The median concentrations of fungi were moderate or low: 3.079 and 3.491 log10 CFU g-1 for strawberries and raspberries, respectively. Fungi of the genus Cladosporium prevailed in the mycobiota of berry fruits, accounting for 84.3% of total isolates in raspberries and 34.8% in strawberries. The occurrence of total aflatoxins was detected in the majority of tested samples (73.2% in raspberries and 70.3% in strawberries) but never exceeded the level of 4.0 µg kg-1 assumed as safe. Deoxynivalenol has been detected only in raspberries with the prevalence of 58.5%. Its median concentration was 242.0 µg kg-1 and in 7 out of 41 samples (17.0%) exceeded the level of 750.0 µg kg-1, assumed as safe.
Conclusions:
Filamentous fungi and mycotoxins occurred in the examined berries at levels that mostly do not represent a health risk for immunocompetent people, but might pose such risk for immuno-compromised and/or atopic consumers.
REFERENCES (74)
1.
Jung Y, Jang H, Matthews KR. Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb Biotechnol. 2014; 7(6): 517–527. https://doi.org/10.1111/1751-7....
2.
Berger CN, Sodha SV, Shaw RK, et al. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol. 2010; 12(9): 2385–2397. https://doi.org/10.1111/j.1462....
3.
Mason-D‘Croz D, Bogard JR, Sulser TB, et al. Gaps between fruit and vegetable production, demand, and recommended consumption at global and national levels: an integrated modelling study. Lancet Planet Health. 2019; 3(7): e318-e329. https://10.1016/S2542-5196(19)....
4.
Balali GI, Yar DD, Afua Dela VG, et al. Microbial contamination, an increasing threat to the consumption of fresh fruits and vegetables in today‘s world. Int J Microbiol. 2020; 2020: 3029295. https://doi.org/10.1155/2020/3....
5.
Jąder K. Consumption of fruits and vegetables in Poland compared with the European Union. PAAAE. 2009: 11(3): 147–153.
6.
Olaimat AN, Holley RA. Factors influencing the microbial safety of fresh produce: a review. Food Microbiol. 2012; 32(1): 1–19. https://doi.org/10.1016/j.fm.2....
7.
Iwu CD, Okoh AI. Preharvest transmission routes of fresh produce associated bacterial pathogens with outbreak potentials: a review. Int J Environ Res Public Health. 2019; 16(22): 4407. https://doi.org/10.3390/ijerph....
8.
Tournas VH, Katsoudas E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int J Food Microbiol. 2005; 105(1): 11–17. https://doi.org/10.1016/j.ijfo....
9.
Alshannaq A, Yu JH. Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Public Health. 2017; 14(6): 632. https://doi.org/10.3390/ijerph....
10.
Ráduly Z, Szabó L, Madar A, et al. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front Microbiol. 2020; 10: 2908. https://doi.org/10.3389/fmicb.....
11.
Reddy KRN, Salleh B, Saad B, et al. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010; 29: 3–26. https://doi.org/10.3109/155695....
12.
Moretti A, Susca A, editors. Mycotoxigenic Fungi. Methods and Protocols. Methods in Molecular Biology 1542. New York: Springer Protocols. Humana Press, Springer; 2017.
13.
Badosa E, Trias R, Parés D, et al. Microbiological quality of fresh fruit nd vegetable products in Catalonia (Spain) using normalised plate counting methods and real time polymerase chain reaction (QPCR). J Sci Food Agric. 2008; 88(4): 605–611. https://doi.org/10.1002/jsfa.3....
14.
Buyukunal SK, Issa G, Aksu F, et al. Microbiological quality of fresh vegetables and fruits collected from supermarkets in Istanbul, Turkey. J Food Nutr Sci. 2015; 3(4): 152–159. https://doi.org/10.11648/j.jfn....
15.
Malarczyk D, Panek J, Frąc M. Alternative molecular-based diagnostic methods of plant pathogenic fungi affecting berry crops-a review. Molecules. 2019; 24(7): 1200. https://doi.org/10.3390/molecu....
16.
Hussein MA, El-Said AHM, Yassein AS. Mycobiota associated with strawberry fruits, their mycotoxin potential and pectinase activity. Mycology. 2020; 11(2): 158–166. https://doi.org/10.1080/215012....
17.
Rigotti S, Viret O, Gindrat D. Fungi from symptomless strawberry plants in Switzerland. Phytopatol Mediterr. 2003; 42: 85–88.
18.
Mouden N, Al Batnan A, Benkirane R, et al. Diversity and distribution f fungi from strawberry plants grown in Gharb-Loukkos (Morocco). Int J Recent Sci Res. 2016; 7(10): 13630–13641.
19.
Abdelfattah A, Wisniewski M, Li Destri Nicosia MG, et al. Metagenomic analysis of fungal diversity on strawberry plants and the effect of management practices on the fungal community structure of aerial organs. PLoS One. 2016; 11(8): e0160470. https://doi.org/10.1371/journa....
20.
Farian E, Wójcik-Fatla A. Diversity and drug resistance of filamentous fungi isolated from the fresh raspberries. Indian J Microbiol. 2022; 62(1): 146–151. https://doi.org/10.1007/s12088....
21.
Fernández-Cruz ML, Mansilla ML, Tadeo JL. Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. J Adv Res. 2010; 1(2): 113–122. https://doi.org/10.1016/j.jare....
22.
Juan C, Oueslati S, Manes J. Evaluation of Alternaria mycotoxins in trawberries: quantification and storage condition. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016; 33(5): 861–868. https://doi.org/10.1080/194400....
23.
Kłapeć T, Cholewa G, Cholewa A, et al. Fungal diversity of root vegetables and soil rhizosphere collected from organic and conventional farms in Eastern Poland. Ann Agric Environ Med. 2018; 25(2): 374–381. https://doi.org/10.26444/aaem/....
24.
Kłapeć T, Wójcik-Fatla A, Farian E, et al. Levels of filamentous fungi and selected mycotoxins in leafy and fruit vegetables and analysis of their potential health risk for consumers. Ann Agric Environ Med. 2021; 28(4): 585–594. https://doi.org/10.26444/aaem/....
25.
EFSA Panel on Contaminants in the Food Chain (CONTAM), Knutsen HK, Alexander J, et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017; 15(9): e04718. https://doi.org/10.2903/j.efsa....
26.
Samson RA, Houbraken J, Frisvad JC, et al. Food and Indoor Fungi. CBS-KNAW, Fungal Biodiversity Centre, 2010.
27.
Watanabe T. Pictorial Atlas of Soil and Seed Fungi. CRC Press, 2010.28. Krzyściak P, Skóra M, Macura AB. Atlas grzybów chorobotwórczych człowieka. 1st ed. MedPharm; 2011.
28.
Krzyściak P, Skóra M, Macura AB. Atlas grzybów chorobotwórczych człowieka. 1st MedPharm; 2011.
29.
Kespohl S, Raulf M. Mould allergens: Where do we stand with molecular allergy diagnostics?: Part 13 of the series Molecular Allergology. Allergo J Int. 2014; 23(4): 120–125. https://doi.org/10.1007/s40629....
30.
Levetin E, Horner WE, Scott JA, et al. Taxonomy of Allergenic Fungi. J Allergy Clin Immunol Pract. 2016; 4(3): 375–385.e1. https://doi.org/10.1016/j.jaip....
31.
European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, L 364/5, 2006.
32.
EFSA Panel on Contaminants in the Food Chain (CONTAM), Schrenk D, Bignami M, et al. Risk assessment of aflatoxins in food. EFSA J. 2020; 18(3): e06040. https://doi.org/10.2903/j.efsa....
33.
Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007; 20(4): 695–704. https://doi.org/10.1128/CMR.00....
34.
Selman M, Pardo A, King TEJr, et al. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012; 186(4): 314–324. https://doi.org/10.1164/rccm.2....
35.
Paterson RRM, Lima N. Filamentous fungal human pathogens from food emphasizing Aspergillus, Fusarium and Mucor. Microorganisms. 2017; 5(3): 44. https://doi.org/10.3390/microo....
36.
Skóra M, Bielecki J, Bulanda M, et al. Fungi of the genus Scopulariopsis – ill-defined human pathogens. Post Mikrobiol. 2015; 54(1): 44–52.
37.
Hu W, Ran Y, Zhuang K, et al. Alternaria arborescens infection in a healthy individual and literature review of cutaneous alternariosis. Mycopathologia. 2015; 179(1–2): 147–152. https://doi.org/10.1007/s11046....
38.
Luo Y, Li J, Zhang X, et al. Characterization of potential pathogenic Cladosporium exposure risks from heating, ventilation and air conditioning (HVAC) in two cities, China. Med Mycol Open Access. 2016; 2: 18. https://doi.org/10.21767/2471-....
39.
Hatvani L, Homa M, Chenthamara K, et al. Agricultural systems as potential sources of emerging human mycoses caused by Trichoderma: a successful, common phylotype of Trichoderma longibrachiatum in the frontline. FEMS Microbiol Lett. 2019; 366(21): fnz246. https://doi.org/10.1093/femsle....
40.
Ostrosky-Zeichner L, Sobel JD. Fungal Infections. Infect Dis Clin North Am. 2016; 30: 143–163. https://doi.org/10.1016/S0891-....
41.
Escrivá L, Oueslati S, Font G, et al. Alternaria mycotoxins in food and feed: an overview. J Food Quality. 2017; ID 1569748(20). https://doi.org/10.1155/2017/1....
42.
Luciano-Rosario D, Keller NP, Jurick WM. Penicillium expansum: iology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol Plant Pathol. 2020; 21: 1391–1404. https://doi.org/10.1111/mpp.12....
43.
Meena M, Gupta SK, Swapnil P, et al. Alternaria toxins: potential virulence factors and genes related to pathogenesis. Front Microbiol. 2017; 8: 1451. https://doi.org/10.3389/fmicb.....
44.
Klewicka E, Sójka M, Ścieszka S, et al. The antimycotic efect of ellagitannins from raspberry (Rubus idaeus L.) on Alternaria alternate ŁOCK 0409. Eur Food Res Technol, 2020; 246: 1341–1349. https://doi.org/10.1007/s00217....
45.
Alwatban MA, Hadi S, Moslem MA. Mycotoxin production in Cladosporium species influenced by temperature regimes. J Pure Appl Microbiol. 2014; 8(5): 4061-4069.
46.
Corrier DE. Mycotoxicosis: mechanisms of immunosuppression. Vet Immunol Immunopathol. 1991; 30(1): 73–87. https://doi.org/10.1016/0165-2....
47.
Alassane-Kpembi I, Kolf-Clauw M, Gauthier T, et al. New insights into mycotoxin mixtures: the toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol Appl Pharmacol. 2013; 272(1): 191–198. https://doi.org/10.1016/j.taap....
48.
Popescu FD. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015; 5(2): 31–50. https://doi.org/10.5662/wjm.v5....
49.
Luccioli S, Malka-Rais J, Nsnuli TM, et al. Clinical reactivity to ingestion challenge with mixed mold extract may be enhanced in subjects sensitized to molds. Allergy Asthma Proc. 2009; 30(4): 433–442. https://doi.org/10.2500/aap.20....
50.
Schütze N, Lehmann I, Bönisch U, et al. Exposure to mycotoxins increases the allergic response in a murine asthma model. Am J Respir Crit Care Med. 2010; 181(11): 1188–1199. https://doi.org/10.1164/rccm.2....
51.
Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010; 23(4): 884–928. https://doi.org/10.1128/CMR.00....
52.
Romano C, Valenti L, Miracco C, et al. Two cases of cutaneous phaeohyphomycosis by Alternaria alternata and Alternaria tenuissima. Mycopathologia. 1997; 137: 65–74. https://doi.org/10.1023/A:1006....
53.
Kieselová K, Gomes T, Santiago F, et al. Emerging cutaneous phaeohyphomycosis caused by Alternaria infectoria. Acta Med Port. 2020; 33(13). https://doi.org/10.20344/amp.1....
54.
Hilmioglu S, Metin DY, Tasbakan M, et al. Skin infection on both legs caused by Acremonium strictum (case report). Ann Saudi Med. 2015; 35(5): 406–408. https://doi.org/10.5144/0256-4....
55.
Egbuta MA, Mwanza M, Babalola OO. Health risks associated with exposure to filamentous fungi. Int J Environ Res Public Health. 2017; 14(7): 719. https://doi.org/10.3390/ijerph....
56.
Machowicz-Matejko E, Furmańczyk A, Zalewska ED. Aspergillus penicillioides Speg. implicated in keratomycosis. Pol J Microbiol. 2018; 67(4): 407–416. https://doi.org/10.21307/pjm-2....
57.
Sadikovic D, Leduc C. Aspergillus penicilliodes. Mold Busters. https://www.bustmold.com/resou... (access: 08.02.2022).
58.
Popp W, Ritschka L, Zwick H, et al. “Berry sorter’s lung” or wine grower’s lung – an exogenous allergic alveolitis caused by Botrytis cinerea spores. Prax Klin Pneumol. 1987; 41(5): 165–169.
59.
Hashimoto S, Tanaka E, Ueyama M, et al. A case report of pulmonary Botrytis sp. infection in an apparently healthy individual. BMC Infect Dis. 2019; 19, 684. https://doi.org/10.1186/s12879....
60.
Nath R, Barua S, Barman J, et al. Subcutaneous mycosis due to Cladosporium cladosporioides and Bipolaris cynodontis from Assam, North-East India and review of published literature. Mycopathologia. 2015; 180(5–6): 379–387. https://doi.org/10.1007/s11046....
61.
Batra N, Kaur H, Mohindra S, et al. Cladosporium sphaerospermum causing brain abscess, a saprophyte turning pathogen: case and review of published reports. J Mycol Med. 2019; 29(2): 180–184. https://doi.org/10.1016/j.mycm....
62.
Habibi A, Safaiefarahani B. Indoor damp surfaces harbor molds with clinical significance. Curr Med Mycol. 2018; 4(3): 1–9. https://doi.org/10.18502/cmm.4....
63.
Stenglein SA. Fusarium poae: A pathogen that needs more attention. J Plant Pathol. 2009; 91(1): 25–36. http://dx.doi.org/10.4454/jpp.....
64.
Merget R, Sander I, Rozynek P, et al. Occupational immunoglobulin E-mediated asthma due to Penicillium camemberti in a dry-sausage packer. Respiration. 2008; 76: 109–111. https://doi.org/10.1159/000097....
65.
Ficociello B, Masciarelli E, Casorri L, et al. The onset of occupational diseases in mushroom cultivation and handling operators: a review. Ital J Mycol. 2019; 48(1): 26–38. https://doi.org/10.6092/issn.2....
66.
Ueno Y, Sato N, Ito T, et al. Chronic toxicity and hepatocarcinogenicity of (+) rugulosin, an anthraquinoid mycotoxin from Penicillium species: preliminary surveys in mice. J Toxicol Sci. 1980; 5(4): 295–302. https://doi.org/10.2131/jts.5.....
67.
Rodríguez-Lobato E, Ramírez-Hobak L, Aquino-Matus JE, et al. Primary cutaneous mucormycosis caused by Rhizopus oryzae: a case report and review of literature. Mycopathologia. 2016; 182 (3–4): 387–392. https://doi.org/10.1007/s11046....
68.
Yilmaz N, Houbraken J, Hoekstra ES, et al. Delimitation and haracterisation of Talaromyces purpurogenus and related species. Pers Mol Phylogeny Evol Fungi. 2012; 29: 39–54. http://dx.doi.org/10.3767/0031....
69.
Aboutalebian S, Mahmoudi S, Okhovat A, et al. Otomycosis due to the rare fungi Talaromyces purpurogenus, Naganishia albida and Filobasidium magnum. Mycopathologia. 2020 Jun; 185(3): 569–575. https://doi.org/10.1007/s11046....
70.
McCormick SP, Stanley AM, Stover NA. Trichothecenes: from simple to complex mycotoxins. Toxins. 2011; 3(7): 802–814. https://doi.org/10.3390/toxins....
71.
Kantarcioglu AS, Celkan T, Yücel A, et al. Fatal Trichoderma harzianum infection in a leukemic pediatric patient. Med Mycol. 2009; 47(2): 207–215. https://doi.org/10.1080/136937....
72.
Weinhold B. “Trilongins” offer insight into mold toxicity. Environ Health Perspect. 2013; 121(2): A44. https://doi.org/10.1289/ehp.12....
73.
Department of Environmental Health and Safety (DEHS), University of Minnesota. Trichothecium spp. https://dehs.umn.edu/trichothe... (access: 08.02.2022).
74.
Liu D. Ulocladium. In: Liu D (Ed) Molecular Detection of Human Fungal Pathogens. CRC Press, Taylor and Francis 2011, pp. 157–160.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.