Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
CASE REPORT
Mycobacterium avium and Klebsiella pneumoniae co-infection in the domestic ferret (Mustela putorius furo) – Case Report
1
Department of Food Hygiene and Public Health Protection, Institute of Veterinary Medicine, University of Life Sciences (SGGW), Warsaw, Poland
2
Department of Microbiology, National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland
3
SKVet Veterinary Clinic, Wrocław, Poland
4
Department of Preclinical Sciences, Institute of Veterinary Medicine, Warsaw University of Life Sciences, Warsaw, Poland
Corresponding author
Anna Didkowska
Department of Food Hygiene and Public Health Protection, Institute of Veterinary Medicine, Warsaw University of Life Sciences (SGGW),, Nowoursynowska 166, 02-787, Warsaw, Poland
Department of Food Hygiene and Public Health Protection, Institute of Veterinary Medicine, Warsaw University of Life Sciences (SGGW),, Nowoursynowska 166, 02-787, Warsaw, Poland
Ann Agric Environ Med. 2024;31(2):298-301
KEYWORDS
TOPICS
ABSTRACT
Introduction and Objective. Pets infected with zoonotic pathogens might become a source of infections for their owners,
especially those who are immuno-compromised. The aim of this report is to describe a case of chronic, untreatable pneumonia
in a domestic ferret.
Materials and method. The subject was a 5-year-old female ferret suffering from recurrent pneumonia. Ante-mortally,
swabs from the nasal cavity, alveolus and throat were collected from the animal. Post-mortally, lesioned organ fragments
were collected. Standard microbiological testing was performed. Additionally, mycobacterial diagnosis including culture
and molecular tests was performed.
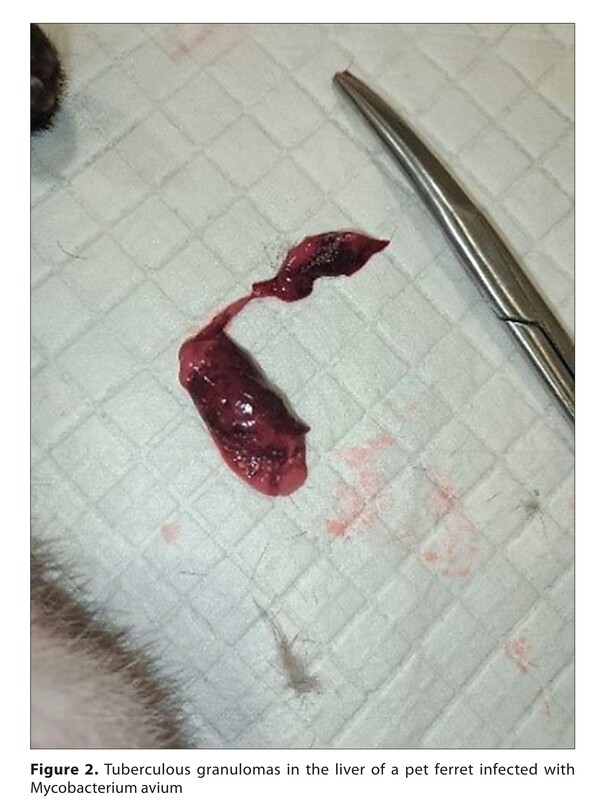
Results. The co-infection of Mycobacterium avium and Klebsiella pneumoniae was microbiologically confirmed.
Conclusions. This case demonstrates the need to pay attention to the possibility of zoonotic pathogens in ferrets.
Veterinarians diagnosing ferrets are potentially exposed to Mycobacteria spp. infections and other pathogens.
REFERENCES (43)
1.
Belser JA, Eckert AM, Tumpey TM, et al. Complexities in Ferret Influenza Virus Pathogenesis and Transmission Models. Microbiol Mol Biol Rev. 2016;80(3):733–744. doi:10.1128/MMBR.00022-16.
2.
Albrecht RA, Liu WC, Sant AJ, et al. Moving Forward: Recent Developments for the Ferret Biomedical Research Model. mBio. 2018;9(4):e01113–18. doi:10.1128/mBio.01113-18.
3.
Horman WSJ, Kedzierska K, Rootes CL, et al. Ferret Interferon (IFN)-Inducible Transmembrane Proteins Are Upregulated by both IFN-α and Influenza Virus Infection. J Virol. 2021;95(14):e0011121. doi:10.1128/JVI.00111-21.
4.
Bannantine JP, Gupta T, Zinniel DK, et al. Use of a Ferret Model to Test Efficacy and Immunogenicity of Live Attenuated Mycobacterium avium subspecies paratuberculosis Vaccines. Methods Mol Biol. 2022;2411:95–104. doi:10.1007/978-1-0716-1888-2_5.
5.
Castanheira de Matos RE, Morrisey JK. Common procedures in the pet ferret. Vet Clin North Am Exot Anim Pract. 2006;9(2):347–365, vii. doi:10.1016/j.cvex.2006.01.003.
6.
Wyre NR. Emerging Zoonotic Diseases in Ferrets. Vet Clin North Am Exot Anim Pract. 2020;23(2):299–308. doi:10.1016/j.cvex.2020.01.012.
7.
Jo WK, de Oliveira-Filho EF, Rasche A, et al. Potential zoonotic sources of SARS-CoV-2 infections. Transbound Emerg Dis. 2021;68(4):1824–1834. doi:10.1111/tbed.13872.
8.
Haider N, Rothman-Ostrow P, Osman AY, et al. COVID-19-Zoonosis or Emerging Infectious Disease? Front Public Health. 2020;8:596944. doi:10.3389/fpubh.2020.596944.
9.
Pramod RK, Nair AV, Tambare PK, et al. Reverse zoonosis of coronavirus disease-19: Present status and the control by one health approach. Vet World. 2021;14(10):2817–2826. doi:10.14202/vetworld.2021.2817-2826.
10.
Račnik J, Kočevar A, Slavec B, et al. Transmission of SARS-CoV-2 from Human to Domestic Ferret. Emerg Infect Dis. 2021;27(9):2450–2453. doi:10.3201/eid2709.210774.
11.
Ramírez-Pizarro F, Silva-de la Fuente C, Hernández-Orellana C, et al. Zoonotic Pathogens in the American Mink in Its Southernmost Distribution. Vector Borne Zoonotic Dis. 2019;19(12):908–914. doi:10.1089/vbz.2019.2445.
12.
Pollock C. Mycobacterial infection in the ferret. Vet Clin North Am Exot Anim Pract. 2012;15(1):121–129, vii. doi:10.1016/j.cvex.2011.09.002.
13.
de Lisle GW, Crews K, de Zwart J, et al. Mycobacterium bovis infections in wild ferrets. NZVet J. 1993;41(3):148–149. doi:10.1080/00480169.1993.35757.
14.
Byrom AE, Caley P, Paterson BM, et al. Feral ferrets (Mustela furo) as hosts and sentinels of tuberculosis in New Zealand. NZVet J. 2015 Jun;63 Suppl 1(sup1):42–53. doi:10.1080/00480169.2014.981314.
15.
Barth SA, Menge C, Hillemann D, et al. Tuberculosis in a pet ferret (Mustela putorius furo). Tierarztl Prax Ausg K Kleintiere Heimtiere. 2020;48(1):50–55. English. doi:10.1055/a-1069-6630.
16.
Xavier Emmanuel F, Seagar AL, Doig C, et al. Human and animal infections with Mycobacterium microti, Scotland. Emerg Infect Dis. 2007;13(12):1924–1927. doi:10.3201/eid1312.061536.
17.
Bezos J, Álvarez-Carrión B, Rodríguez-Bertos A, et al. Evidence of disseminated infection by Mycobacterium avium subspecies hominissuis in a pet ferret (Mustela putorius furo). Res Vet Sci. 2016;109:52–55. doi:10.1016/j.rvsc.2016.09.013.
18.
Mentré V, Bulliot C. A Retrospective Study of 17 Cases of Mycobacteriosis in Domestic Ferrets (Mustela Putorius furo) between 2005 and 2013. J Exot Pet Med. 2015;24(3):340–349. doi:10.1053/j.jepm.2015.06.019.
19.
Lunn JA, Martin P, Zaki S, et al. Pneumonia due to Mycobacterium abscessus in two domestic ferrets (Mustelo putorius furo). Aust Vet J. 2005;83(9):542–546. doi:10.1111/j.1751-0813.2005.tb13325.x.
20.
Davendralingam N, Davagnanam I, Stidworthy MF, et al. Transmission of Mycobacterium xenopi to a pet albino ferret (Mustela putorius furo) from a domestic aquarium. Vet Rec. 2017;181(7):169. doi:10.1136/vr.104250.
21.
De Lorenzi G, Kamphuisen K, Biscontini G, et al. Mycobacterium genavense Infection in a Domestic Ferret (Mustela putorius furo). Top Companion Anim Med. 2018;33(4):119–121. doi:10.1053/j.tcam.2018.10.001.
22.
Dequéant B, Pascal Q, Bilbault H, et al. Identification of Mycobacterium genavense natural infection in a domestic ferret. J Vet Diagn Invest. 2019;31(1):133–136. doi:10.1177/1040638718812137.
23.
van Ingen J, Turenne CY, Tortoli E, et al. A definition of the Mycobacterium avium complex for taxonomical and clinical purposes, a review. Int J Syst Evol Microbiol. 2018;68(11):3666–3677. https://doi.org/10.1099/ijsem.....
24.
Ludwig E, Reischl U, Holzmann T, et al. Risk for Mycobacterium celatum infection from ferret. Emerg Infect Dis. 2011;17(3):553–555. doi:10.3201/eid1703.100969.
25.
Iyengar KP, Nadkarni JB, Gupta R, et al. Mycobacterium chelonae hand infection following ferret bite. Infection. 2013;41(1):237–241. doi:10.1007/s15010-012-0309-7.
26.
Jones JW, Pether JV, Rainey HA, et al. Recurrent Mycobacterium bovis infection following a ferret bite. J Infect. 1993;26(2):225–226. doi:10.1016/0163-4453(93)93220-x.
27.
Didkowska A, Orłowska B, Krajewska-Wędzina M, et al. Microbiological and molecular monitoring for bovine tuberculosis in the Polish population of European bison (Bison bonasus). Ann Agric Environ Med. 2021;28(4):575–578. doi:10.26444/aaem/130822.
28.
Jian-Li W, Yuan-Yuan S, Shou-Yu G, et al. Serotype and virulence genes of Klebsiella pneumoniae isolated from mink and its pathogenesis in mice and mink. Sci Rep. 2017;7(1):17291. doi:10.1038/s41598-017-17681-8.
29.
Valencia-Bacca J, Silva MM, Cerdeira L, et al. Detection and Whole-Genome Analysis of a High-Risk Clone of Klebsiella pneumoniae ST340/CG258 Producing CTX-M-15 in a Companion Animal. Microb Drug Resist. 2020;26(6):611–615. doi:10.1089/mdr.2019.0190.
30.
Fenollar F, Mediannikov O, Maurin M, et al. Mink, SARS-CoV-2, and the Human-Animal Interface. Front Microbiol. 2021;12:663815. doi:10.3389/fmicb.2021.663815.
31.
Krajewska-Wędzina M, Radulski Ł, Waters WR, et al. Mycobacterium bovis Transmission between Cattle and a Farmer in Central Poland. Pathogens. 2022;11(10):1170. doi:10.3390/pathogens11101170.
32.
Kaczmarkowska A, Didkowska A, Kwiecień E, et al. The Mycobacterium avium complex – an underestimated threat to humans and animals. Ann Agric Environ Med. 2022;29(1):22–27. doi:10.26444/aaem/136398.
33.
Olea-Popelka F, Muwonge A, Perera A, et al. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis-a call for action. Lancet Infect Dis. 2017;17(1):e21-e25. doi:10.1016/S1473-3099(16)30139-6.
34.
Dow CT, Alvarez BL. Mycobacterium paratuberculosis zoonosis is a One Health emergency. Ecohealth. 2022;19(2):164–174. doi:10.1007/s10393-022-01602-x.
35.
Heidary M, Nasiri MJ, Mirsaeidi M, et al. Mycobacterium avium complex infection in patients with human immunodeficiency virus: A systematic review and meta-analysis. J Cell Physiol. 2019;234(7):9994–10001. doi:10.1002/jcp/27859.
36.
Ratnatunga CN, Lutzky VP, Kupz A, et al. The Rise of Non-Tuberculosis Mycobacterial Lung Disease. Front Immunol. 2020;11:303. doi:10.3389/fimmu.2020.00303.
37.
Kwon YS, Koh WJ, Daley CL. Treatment of Mycobacterium avium Complex Pulmonary Disease. Tuberc Respir Dis. 2019;82(1):15–26. doi:10.4046/trd.2018.0060.
38.
Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to nontuberculous mycobacteria. Int J Infect Dis. 2016;45:123–134. doi:10.1016/j.ijid.2016.03.006.
39.
Slany M, Ulmann V, Slana I. Avian Mycobacteriosis: Still Existing Threat to Humans. Biomed Res Int. 2016;4387461:1–12. doi:10.1155/2016/4387461.
40.
Cowman S, van Ingen J, Griffith DE, et al. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J. 2019;54(1):1900250. doi:10.1183/13993003.00250-2019.
41.
Mintz MJ, Lukin DJ. Mycobacterium avium subspecies paratuberculosis (MAP) and Crohn’s disease: the debate continues. Transl Gastroenterol Hepatol. 2023 Jul 25;8:28. doi:10.21037/tgh-23-16.
42.
Agrawal G, Aitken J, Hamblin H, et al. Putting Crohn’s on the MAP: Five Common Questions on the Contribution of Mycobacterium avium subspecies paratuberculosis to the Pathophysiology of Crohn’s Disease. Dig Dis Sci. 2021 Feb;66(2):348–358. doi:10.1007/s10620-020-06653-0.
43.
Honap S, Johnston E, Agrawal G, et al. Anti-Mycobacterium paratuberculosis (MAP) therapy for Crohn’s disease: an overview and update. Frontline Gastroenterol. 2020 Jul 28;12(5):397–403. doi:10.1136/flgastro-2020-101471.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.