Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Invasive and native mosquitoes in Europe, including Poland, as vectors transmitting pathogens – implications for wellbeing of Armed Forces
1
Molecular biology laboratory, Military Institute of Aviation Medicine, Warsaw, Poland
2
Department of Biomedical Sciences, Józef Piłsudski University of Physical Education, Warsaw, Poland
Corresponding author
Katarzyna Komar
Molecular biology laboratory, Military Institute of Aviation Medicine, Warsaw, Poland
Molecular biology laboratory, Military Institute of Aviation Medicine, Warsaw, Poland
Ann Agric Environ Med. 2025;32(2):167-172
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Mosquitoes play a crucial role as vectors transmitting various pathogens, including parasites and viruses, which cause serious diseases worldwide, such as malaria, dengua or West Nile virus. Both invasive and native species are capable of spreading tropical diseases, which endanger troops stationing in areas rich in mosquitos. The aim of the review is to analyze current knowledge about the distribution of mosquito species in Europe, including Poland, and the factors influencing their presence.
Review methods:
A systematic literature review was conducted using databases such as PubMed and Google Scholar. The search included key words related to mosquitoes, vector-borne diseases, and climate change. Data were supplemented with information from the websites of the World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC).
Brief description of the state of knowledge:
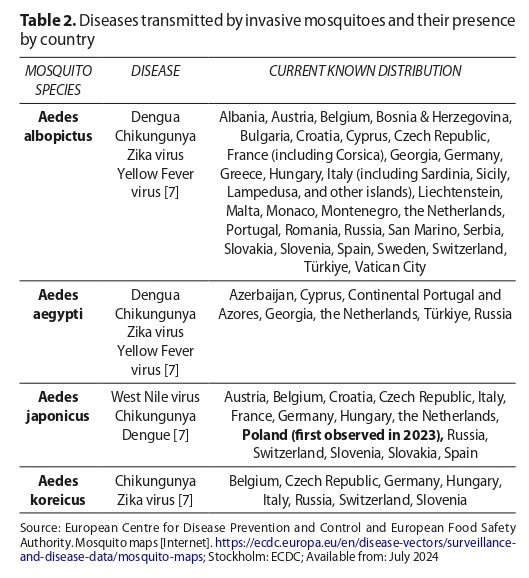
Invasive mosquitoes, such as Aedes albopictus, have been spreading in Europe since the 1990s. Rising temperatures and global movement of people and goods are the main driving forces of this phenomenon. Even native European mosquito species, like Culex spp., are able to transmit tropical diseases. The European Mosquito Control Association is developing mosquito control programmes but they are not sufficiently implemented.
Summary:
Mosquitoes transmit various viral and parasitic diseases. The presence of invasive exotic mosquitoes in Europe is linked to developments in transportation and climate change. Rising temperatures enable mosquitoes to adapt to new regions previously free of them. This issue requires effective protective measures and competent personnel to implement vector control methods.
Mosquitoes play a crucial role as vectors transmitting various pathogens, including parasites and viruses, which cause serious diseases worldwide, such as malaria, dengua or West Nile virus. Both invasive and native species are capable of spreading tropical diseases, which endanger troops stationing in areas rich in mosquitos. The aim of the review is to analyze current knowledge about the distribution of mosquito species in Europe, including Poland, and the factors influencing their presence.
Review methods:
A systematic literature review was conducted using databases such as PubMed and Google Scholar. The search included key words related to mosquitoes, vector-borne diseases, and climate change. Data were supplemented with information from the websites of the World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC).
Brief description of the state of knowledge:
Invasive mosquitoes, such as Aedes albopictus, have been spreading in Europe since the 1990s. Rising temperatures and global movement of people and goods are the main driving forces of this phenomenon. Even native European mosquito species, like Culex spp., are able to transmit tropical diseases. The European Mosquito Control Association is developing mosquito control programmes but they are not sufficiently implemented.
Summary:
Mosquitoes transmit various viral and parasitic diseases. The presence of invasive exotic mosquitoes in Europe is linked to developments in transportation and climate change. Rising temperatures enable mosquitoes to adapt to new regions previously free of them. This issue requires effective protective measures and competent personnel to implement vector control methods.
ACKNOWLEDGEMENTS
The research was funded in 2021–2025 by the Ministry of Health in Warsaw, Poland, as part of the National Health Programme under Agreement No. 364/2021/DA of 29 November 2021.
REFERENCES (50)
1.
Wilson AJ, Morgan ER, Booth M, et al. What is a vector? Philosophical Transactions of the Royal Society B: Biological Sci. 2017;372(1719). doi:10.1098/rstb.2016.0085.
3.
Franklinos LHV, Jones KE, Redding DW, et al. The effect of global change on mosquito-borne disease. Lancet Infect Dis. 2019;19(9):e302-e312. doi:10.1016/S1473-3099(19)30161-6.
4.
Borecka A, Szczypek M, Pabin A, et al. Impact of tick-borne pathogens on the health risk of soldiers. Ann Agric Environ Med. 2023;30(2):211–216. doi:10.26444/aaem/159702.
5.
Benelli G, Beier JC. Current vector control challenges in the fight against malaria. Acta Trop. 2017;174:91–96. doi:10.1016/j.actatropica.2017.06.028.
7.
Giunti G, Becker N, Benelli G. Invasive mosquito vectors in Europe: From bioecology to surveillance and management. Acta Trop. 2023;239. doi:10.1016/j.actatropica.2023.106832.
8.
Rajput R, Sharma J, Nair MT, et al. Regulation of Host Innate Immunity by Non-Coding RNAs During Dengue Virus Infection. Front Cell Infect Microbiol. 2020;10. doi:10.3389/fcimb.2020.588168.
9.
Jawień P, Pfitzner WP, Schaffner F, et al. Mosquitoes (Diptera: Culicidae) of Poland: An Update of Species Diversity and Current Challenges. Insects. 2024;15(5):353. doi:10.3390/insects15050353.
10.
Kuna A, Gajewski M, Biernat B. Selected arboviral diseases imported to Poland – Current state of knowledge and perspectives for research. Ann Agric Environ Med. 2019;26(3):385–391. doi:10.26444/aaem/102471.
11.
Zakład Epidemiologii Chorób Zakaźnych i Nadzoru NIZP-PZH. Zachorowania Na Wybrane Choroby Zakaźne w Polsce Od 1 Stycznia Do 31 Grudnia 2023 r.
12.
Medlock JM, Hansford KM, Schaffner F, et al. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector-Borne Zoonotic Dis. 2012;12(6):435–447. doi:10.1089/vbz.2011.0814.
13.
Ligsay A, Telle O, Paul R. Challenges to mitigating the urban health burden of mosquito-borne diseases in the face of climate change. Int J Environ Res Public Health. 2021;18(9). doi:10.3390/ijerph18095035.
14.
El-Sayed A, Kamel M. Climatic changes and their role in emergence and re-emergence of diseases. Environ Sci Poll Res. 2020;27(18):22336–22352. doi:10.1007/s11356-020-08896-w.
15.
Vogels CBF, Göertz GP, Pijlman GP, et al. Vector competence of European mosquitoes for west Nile virus. Emerg Microbes Infect. 2017;6(11). doi:10.1038/emi.2017.82.
16.
Gajda E, Krzowski Ł, Kowalczuk K, et al. Influence of mosquito-borne biological agents on health risks among soldiersand military personnel. Ann Agric Environ Med. 2023;30(1):2–8. doi:10.26444/aaem/155003.
17.
Sauer FG, Lange U, Schmidt-Chanasit J, et al. Overwintering Culex torrentium in abandoned animal burrows as a reservoir for arboviruses in Central Europe. One Health. 2023;16. doi:10.1016/j.onehlt.2023.100572.
18.
Weitzel T, Jawień P, Rydzanicz K, et al. Culex pipiens s.l. and Culex torrentium (Culicidae) in Wrocław area (Poland): occurrence and breeding site preferences of mosquito vectors. Parasitol Res. 2015;114(1):289–295. doi:10.1007/s00436-014-4193-1.
19.
Lühken R, Brattig N, Becker N. Introduction of invasive mosquito species into Europe and prospects for arbovirus transmission and vector control in an era of globalization. Infect Dis Poverty. 2023;12(1). doi:10.1186/s40249-023-01167-z.
20.
Moutinho S, Rocha J, Gomes A, et al. Spatial Analysis of Mosquito-Borne Diseases in Europe: A Scoping Review. Sustainability (Switzerland). 2022;14(15). doi:10.3390/su14158975.
21.
Wałęka M, Wójcicka P, Żakowska D. Asian tiger mosquito (Aedes albopictus) as a potential vector of diseases and a threat to public health in Poland. Environ Med. 2023:26–31. doi:10.26444/ms/169855.
22.
Rudolf I, Blažejová H, Straková P, et al. The invasive Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in the Czech Republic: Repetitive introduction events highlight the need for extended entomological surveillance. Acta Trop. 2018;185:239–241. doi:10.1016/j.actatropica.2018.05.020.
23.
Bakran-Lebl K, Zittra C, Harl J, et al. Arrival of the Asian tiger mosquito, Aedes albopictus (Skuse, 1895) in Vienna, Austria and initial monitoring activities. Transbound Emerg Dis. 2021;68(6):3145–3150. doi:10.1111/tbed.14169.
24.
European Centre for Disease Prevention and Control. Mosquito maps. ECDC https://www.ecdc.europa.eu/en/... (access: 2024.05.27).
25.
Becker N, Geier M, Balczun C, et al. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res. 2013;112(4):1787–1790. doi://10.1007/s00436-012-3230-1.
26.
Lillepold K, Rocklöv J, Liu-Helmersson J, et al. More arboviral disease outbreaks in continental Europe due to the warming climate? J Travel Med. 2019;26(5). doi:10.1093/jtm/taz017.
27.
Paula Hashim Z, Aguilera-Cruz J, Luke-Currier A, et al. On Urgently Tackling the Mosquito-Borne Diseases in the European Union. South East Eur J Public Health. 2023; 56–78. https://doi.org/10.56801/seejp....
28.
Kuna A, Szostakowska B, Nahorski WL, et al. An attempt to estimate the minimal number of Poles infected and treated for malaria in Poland and abroad. Int Marit Health. 2015;66(4):233–237. doi:10.5603/IMH.2015.0044.
29.
Goljan J, Myjak P, Nahorski W, et al. Dengue antibodies in Polish travellers returning from the tropics. Evaluation of serological tests. Int Marit Health. 2010;61:36–40.
30.
Bętkowska A, Hanke J, Krankowska D, et al. Challenges in diagnosing fever in traveler returning from tropical ares – is it dengue or chikungunya? Case report. Przegl Epidemiol. 2023;76(4):450–457. doi:10.32394/pe.76.42.
31.
Shi H, Yu X, Cheng G. Impact of the microbiome on mosquito-borne diseases. Protein Cell. 2023;14(10):743–761. doi:10.1093/procel/pwad021.
32.
Stępień M. Malaria in Poland in 2014–2018. Przegl Epidemiol. 2019;73(2):201–209. doi:10.32394/pe.73.19.
33.
Ibáñez-Justicia A, Smitz N, Den Hartog W, et al. Detection of exotic mosquito species (Diptera: Culicidae) at international airports in europe. Int J Environ Res Public Health. 2020;17(10). doi:10.3390/ijerph17103450.
34.
Johnson N, de Marco MF, Giovannini A, et al. Emerging mosquito-borne threats and the response from European and Eastern Mediterranean countries. Int J Environ Res Public Health. 2018;15(12). doi:10.3390/ijerph15122775.
35.
Cholewiński M, Derda M, Hadaś E. Parasitic diseases in humans transmitted by vectors. Ann Parasitol. 2015;61(3):137–157. doi:10.17420/ap6103.01.
36.
Moser SK, Barnard M, Frantz RM, et al. Scoping review of Culex mosquito life history trait heterogeneity in response to temperature. Parasit Vectors. 2023;16(1). doi:10.1186/s13071-023-05792-3.
37.
Epstein PR. Climate change and emerging infectious diseases. Microbes Infect. 2001;3:747–754.
38.
George AM, Ansumana R, de Souza DK, et al. Climate change and the rising incidence of vector-borne diseases globally. Int J Infect Dis. 2024;139:143–145. doi:10.1016/j.ijid.2023.12.004.
39.
Wu X, Lu Y, Zhou S, et al. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ Int. 2016;86:14–23. doi:10.1016/j.envint.2015.09.007.
40.
Beck-Johnson LM, Nelson WA, Paaijmans KP, et al. The effect of temperature on Anopheles mosquito population dynamics and the potential for malaria transmission. PLoS One. 2013;8(11). doi:10.1371/journal.pone.0079276.
41.
Erazo D, Grant L, Ghisbain G, et al. Contribution of climate change to the spatial expansion of West Nile virus in Europe. Nat Commun. 2024;15(1). doi:10.1038/s41467-024-45290-3.
42.
Senior K. Climate change and infectious disease: a dangerous liaison? The Lancet. 2008:92–93. doi:10.1016/s1473-3099(08)70008-2.
43.
Vicente-Serrano SM, Peña-Angulo D, Beguería S, et al. Global drought trends and future projections. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2022;380(2238). doi:10.1098/rsta.2021.0285.
44.
Ogden NH, Lindsay LR. Effects of Climate and Climate Change on Vectors and Vector-Borne Diseases: Ticks Are Different. Trends Parasitol. 2016;32(8):646–656. doi:10.1016/j.pt.2016.04.015.
45.
4European Centre for Disease Prevention and Control. Guidelines for the Surveillance of Invasive Mosquitoes in Europe. European Centre for Disease Prevention and Control; 2012.
46.
Centers for Disease Control and Prevention. Genetically Modified Mosquitoes. CDC https://www.cdc.gov/mosquitoes... (access: 2024.06.01).
47.
O’Donnel Francis L, Fan M, Stahlman S. Surveillance for Vector-borne Diseases Among Active and Reserve Component Service Members, U.S. Armed Forces, 2016–2020. MSMR. 2021;28(02):11–16.
48.
Stidham RA, Cole R, Mabila SL. The four most frequently diagnosed vector-borne diseases among service member and non-service member beneficiaries in the geographic combatant commands, 2010–2022. MSMR. 2024;31(1):14–16. doi:10.1126/science.7043737.
49.
Korzeniewski K. Problemy zdrowotne long-term travelers na przykładzie żołnierzy Polskich Kontyngentów Wojskowych w aspekcie praktyki lekarza rodzinnego. Fam Med Primary Care Rev. 2013;15(3):454–457.
50.
Kondrusik M, Zajkowska J, Pancewicz S, et al. Obecność Przeciwciał Reagujących z Antygenem Wirusa Zachodniego Nilu (WNV) Wśród Mieszkańców Województw Podlaskiego i Świętokrzyskiego. Przegl Epidemiol. 2007;61(2):409–416.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.