Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Injecting drug users, MSM and people at the older age should be routinely tested for HCV in Poland – data derived from a post-exposure prophylaxis population
1
Medical University, Warsaw, Poland
2
SWPS University of Sciences and Humanities, Warsaw, Poland
Corresponding author
Ann Agric Environ Med. 2021;28(4):633-638
KEYWORDS
professional exposurepost – exposure prophylaxisrisk factors for HCV infectionHCV testingnon-professional exposure
TOPICS
ABSTRACT
Objective:
The aim of the study was to identify risk factors for HCV infection and thus identify groups for routine HCV testing in the group of people consulted for post-exposure prophylaxis (PEP).
Material and methods:
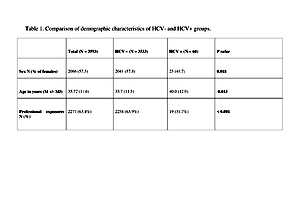
A retrospective analysis was performed of cross-sectional data available from consultations due to post-exposure prophylaxis in HIV Out-patient Clinic and Emergency Department (ED) of Hospital for Infectious Diseases in Warsaw, Poland. Data were obtained from the electronic database, from 2008-o 2016. For statistical analysis, χ2 and t-tests were used for group comparisons, as appropriate. A total of 3,593 persons were included in the study, 60 (1.7%) were anti-HCV positive. In the first step, univariate models were estimated for each of predictors separately.
Results:
The results showed that odds of infection are significantly higher in males (OR = 1.92), people after non-professional exposure (OR = 3.82), and increase with age (OR = 1.03). In the next step, a multivariate logistic model was fitted in the group of participants after non-professional exposure with gender, age, and route of exposure as predictors. Obtained results revealed significantly higher odds of infection, both in IDU (OR = 162.6) and gender exposure (OR = 3.59) groups. After including routes of exposure, effects of age remained significant (OR = 1.05), while the effects of gender did not (OR = 1.12)
Conclusions:
Based on the study results, it is recommended that routine testing for HCV should be provided for people at older age, and for individual with behavioural risk factors, such as history of injecting drus use or sexual exposure, particularly among men having sex with men (MSM)
The aim of the study was to identify risk factors for HCV infection and thus identify groups for routine HCV testing in the group of people consulted for post-exposure prophylaxis (PEP).
Material and methods:
A retrospective analysis was performed of cross-sectional data available from consultations due to post-exposure prophylaxis in HIV Out-patient Clinic and Emergency Department (ED) of Hospital for Infectious Diseases in Warsaw, Poland. Data were obtained from the electronic database, from 2008-o 2016. For statistical analysis, χ2 and t-tests were used for group comparisons, as appropriate. A total of 3,593 persons were included in the study, 60 (1.7%) were anti-HCV positive. In the first step, univariate models were estimated for each of predictors separately.
Results:
The results showed that odds of infection are significantly higher in males (OR = 1.92), people after non-professional exposure (OR = 3.82), and increase with age (OR = 1.03). In the next step, a multivariate logistic model was fitted in the group of participants after non-professional exposure with gender, age, and route of exposure as predictors. Obtained results revealed significantly higher odds of infection, both in IDU (OR = 162.6) and gender exposure (OR = 3.59) groups. After including routes of exposure, effects of age remained significant (OR = 1.05), while the effects of gender did not (OR = 1.12)
Conclusions:
Based on the study results, it is recommended that routine testing for HCV should be provided for people at older age, and for individual with behavioural risk factors, such as history of injecting drus use or sexual exposure, particularly among men having sex with men (MSM)
Pyziak-Kowalska KA, Bielecki M, Horban A, Kowalska J. Injecting drug users, MSM and people at the older age should be routinely tested for HCV in Poland – data derived from the post-exposure prophylaxis population. Ann Agric Environ Med.doi: 10.26444/aaem/131119
REFERENCES (54)
1.
Hepatitis C by the Numbers: Facts, Stats, and You (Infographic) [Available from: https://www.healthline.com/hea....
2.
Hepatitis B and C epidemiology in selected population groups in the EU/EEA [Available from: https://www.ecdc.europa.eu/sit....
3.
Chevaliez S. Strategies for the improvement of HCV testing and diagnosis. Expert review of anti-infective therapy. 2019; 17(5): 341–7.
4.
Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. The lancet HIV. 2016; 3(8): e361-e87.
5.
Kowalska JD, Wojcik G, Rutkowski J, Ankiersztejn-Bartczak M, Siewaszewicz E. Modelling the cost-effectiveness of HIV care shows a clear benefit when transmission risk is considered in the calculations – A message for Central and Eastern Europe. PloS one. 2017; 12(11): e 0186131.
6.
EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015; 63(1): 199–236.
7.
Aspinall EJ, Doyle JS, Corson S, Hellard ME, Hunt D, Goldberg D, et al. Targeted hepatitis C antibody testing interventions: a systematic review and meta-analysis. European journal of epidemiology. 2015; 30(2): 115–29.
8.
Joore IK, Arts DL, Kruijer MJ, Moll van Charante EP, Geerlings SE, Prins JM, et al. HIV indicator condition-guided testing to reduce the number of undiagnosed patients and prevent late presentation in a high-prevalence area: a case-control study in primary care. Sexually transmitted infections. 2015; 91(7): 467–72.
9.
Pyziak-Kowalska KA, Kowalska J, Horban A. Rationales for indicator condition-based HIV testing data from the Hospital for Infectious Diseases in Warsaw – one-year observation. HIV & AIDS Review International Journal of HIV-Related Problems. 2017; 16(3): 191–4.
10.
Raciborski F, Gujski M, Kłak A, Gierczyński J. HCV in Poland – strategy to solve the health problem and actions in perspective 2015–2016. 2015 [Available from: https://www.researchgate.net/p....
12.
NIPH-NIH. Infectious diseases and poisonings in Poland in 2018 [Available from: http://wwwold.pzh.gov.pl/oldpa....
13.
Flisiak R, Halota W, Tomasiewicz K, Kostrzewska K, Razavi HA, Gower EE. Forecasting the disease burden of chronic hepatitis C virus in Poland. European journal of gastroenterology & hepatology. 2015; 27(1): 70–6.
14.
Ganczak M, Korzen M, Szych Z. Seroprevalence of hepatitis C virus infection among surgical nurses, their patients and blood donation candidates in Poland. The Journal of hospital infection. 2012; 82(4): 266 –70.
15.
Flisiak R, Halota W, Horban A, Juszczyk J, Pawlowska M, Simon K. Prevalence and risk factors of HCV infection in Poland. European journal of gastroenterology & hepatology. 2011; 23(12): 1213–7.
16.
Rosińska M, Parda N, Kołakowska A, Godzik P, Zakrzewska K, Madaliński K, et al. Factors associated with hepatitis C prevalence differ by the stage of liver fibrosis: A cross-sectional study in the general population in Poland, 2012–2016. PloS one. 2017; 12(9): e0185055-e.
17.
Grzeszczuk A, Wandalowicz AD, Jaroszewicz J, Flisiak R. Prevalence and Risk Factors of HCV/HIV Co-Infection and HCV Genotype Distribution in North-Eastern Poland. Hepat Mon. 2015; 15(7): e2774 0 -e.
18.
Czerwinski J, Malanowski P, Wasiak D, Pszenny A, Gutowska D, Kwiatkowski A, et al. Viral hepatitis B and C markers in the population of deceased donors in Poland. Transplantation proceedings. 2007; 39 (9): 2 6 9 5 –7.
19.
Czerwinski M, Grabarczyk P, Stepien M, Kubicka-Russel D, Tkaczuk K, Brojer E, et al. What weighs more-low compliance with self-deferral or minor medical procedures? Explaining the high rate of hepatitis C virus window-period donations in Poland. Transfusion. 2017; 57(8): 1998–2006.
20.
Gore C, Hicks J, Deelder W. Funding the elimination of viral hepatitis: donors needed. The lancet Gastroenterology & hepatology. 2017; 2(12): 843–5.
21.
Brouard C, Le Strat Y, Larsen C, Jauffret-Roustide M, Lot F, Pillonel J. The undiagnosed chronically-infected HCV population in France. Implications for expanded testing recommendations in 2014. PloS one. 2015; 10(5): e0126920.
22.
Guardigni V, Morieri ML, Segala D, Sighinolfi L. Disease Progression in HIV Late Presenters: the Role of HIV Clinical Indicator Diseases Prior to HIV Diagnosis. Current HIV research. 2016; 14(4): 346–53.
23.
Flisiak R, Frankova S, Grgurevic I, Hunyady B, Jarcuska P, Kupčinskas L, et al. How close are we to hepatitis C virus elimination in Central Europe? Clinical and Experimental Hepatology. 2020; 6(1): 1–8.
25.
Organization WH. Global Hepatitis Report 2017 [04.09.2019.]. Available from: http://apps.who.int/iris/bitst....
26.
Addiction EMCf DaD. Rapid comunication: Drug-related infectious disease in Europe: update from the EMCDDA expert network Lisbon: EMCDDA; 2017 [Available from: http://www.emcdda.europa.eu/ra....
27.
Control ECf DPa. Systematic review on hepatitis B and C prevalence i the EU/EEA Stocholm: ECDC; 2016 [Available from: https://ecdc.europa.eu/sites/p....
28.
Rosińska M, Sierosławski J, Wiessing L. High regional variability of HIV, HCV and injecting risks among people who inject drugs in Poland: comparing a cross-sectional bio-behavioural study with case-based surveillance. BMC infectious diseases. 2015; 15: 83.
29.
Wysocki MJ, Zieliński A, Gierczyński R (ed.). NIZP-PZH Warsaw 2016. p. 61–72 [Available from: http://www.jestemswiadom.org/p....
30.
Sierosławski J, Dąbrowska K. Zapobieganie HCV wśród użytkowników narkotyków. In: Projekt KIK/35. Zapobieganie zakażeniom HCV jako przykład zintegrowanych działań w zdrowiu publicznym na rzecz ograniczenia zakażeń krwiopochodnych w Polsce.
31.
Waheed Y. Transition from millennium development goals to sustainable development goals and hepatitis. Pathogens and global health. 2015; 109(8): 353.
32.
Rafik MM, El Shazly Y, Abbas AA, Abd Elhady W, Ragab D, AlShennawy D. Sexual Transmission of HCV in Heterologous Monogamous Spouses. Journal of sexually transmitted diseases. 2014; 2014: 140640.
33.
Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology (Baltimore, Md). 2010; 52(4): 1497–505.
34.
Grady BP, Prins M, van der Loeff MS. The sexual transmission rate of HCV among heterosexual couples. Hepatology (Baltimore, Md). 2013; 58(5): 1865–6.
35.
Duffell EF, Hedrich D, Mardh O, Mozalevskis A. Towards elimination of hepatitis B and C in European Union and European Economic Area countries: monitoring the World Health Organization’s global health sector strategy core indicators and scaling up key interventions. Eurosurveillance. 2017; 22(9).
36.
Cainelli F. Hepatitis C virus and human immunodeficiency virus transmission routes: Differences and similarities. World journal of hepatology. 2013; 5(5): 234–6.
37.
Kouyos RD, Rauch A, Boni J, Yerly S, Shah C, Aubert V, et al. Clustering of HCV coinfections on HIV phylogeny indicates domestic and sexual transmission of HCV. International journal of epidemiology. 2014; 43(3): 887–96.
38.
Chan DPC, Sun H-Y, Wong HTH, Lee S-S, Hung C-C. Sexually acquired hepatitis C virus infection: a review. International Journal of Infectious Diseases. 2016; 49: 47–58.
39.
Vaux S, Chevaliez S, Saboni L, Sauvage C, Sommen C, Barin F, et al. Prevalence of hepatitis C infection, screening and associated factors among men who have sex with men attending gay venues: a cross-sectional survey (PREVAGAY), France, 2015. BMC infectious diseases. 2019; 19(1): 315.
40.
Nijmeijer BM, Koopsen J, Schinkel J, Prins M, Geijtenbeek TB. Sexually transmitted hepatitis C virus infections: current trends, and recent advances in understanding the spread in men who have sex with men. Journal of the International AIDS Society. 2019; 22(S6): e25348.
41.
Parczewski M, Cielniak I, Kordek J, Aksak-Wąs B, Urbańska A, Leszczyszyn-Pynka M, et al. Transmission Networks of HCV Genotype 1a Enriched With Pre-existing Polymorphism Q80K Among HIV-Infected Patients With Acute Hepatitis C in Poland. Journal of acquired immune deficiency syndromes. 2018; 77(5): 514–22.
42.
Transmission Networks of HCV Genotype 1a Enriched With Pre-existing Polymorphism Q80K Among HIV-Infected Patients With Acute Hepatitis C in Poland: Erratum. Journal of acquired immune deficiency syndromes. 2019; 81(1): e27.
43.
Committee on a National Strategy for the Elimination of Hepatitis B, Board on Population H, Public Health P, Health, Medicine D, National Academies of Sciences E, et al. In: Buckley GJ, Strom BL, editors. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. Washington (DC): National Academies Press (US). Copyright 2016 by the National Academy of Sciences. All rights reserved.; 2016.
44.
Grebely J, Bilodeau M, Feld JJ, Bruneau J, Fischer B, Raven JF, et al. The Second Canadian Symposium on hepatitis C virus: a call to action. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2013; 27(11): 627–32.
45.
Neukam K, Ridruejo E, Perez P, Campos RH, Martinez AP, Di Lello FA. Prevalence of hepatitis C virus infection according to the year of birth:identification of risk groups. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2018; 37(2): 247–54.
46.
Walewska-Zielecka B, Religioni U, Juszczyk G, Wawrzyniak ZM, Czerw A, Soszyński P, et al. Anti-hepatitis C virus seroprevalence in the working age population in Poland, 2004 to 2014. Eurosurveillance. 2017; 22(2).
47.
Hartleb M, Gutkowski K, Zejda JE, Chudek J, Wiecek A. Serological prevalence of hepatitis B virus and hepatitis C virus infection in the elderly population: Polish nationwide survey--PolSenior. European journal of gastroenterology & hepatology. 2012; 24(11): 1288–95.
48.
Sakem B, Madaliński K, Nydegger U, Stępień M, Godzik P, Kołakowska A, et al. Hepatitis C virus epidemiology and prevention in Polish and Swiss population – similar and contrasting experiences. Annals of Agricultural and Environmental Medicine. 2016; 23(3): 425–31.
49.
Coppola N, De Pascalis S, Onorato L, Calo F, Sagnelli C, Sagnelli E. Hepatitis B virus and hepatitis C virus infection in healthcare workers. World journal of hepatology. 2016; 8(5): 273–81.
50.
Slusarczyk J, Malkowski P, Bobilewicz D, Juszczyk G. Cross-sectional, anonymous screening for asymptomatic HCV infection, immunity to HBV, and occult HBV infection among health care workers in Warsaw, Poland. Przeglad epidemiologiczny. 2012; 66(3): 445–51.
51.
Rybacki M, Piekarska A, Wiszniewska M, Walusiak-Skorupa J. Hepatitis B and C infection: is it a problem in Polish healthcare workers? International journal of occupational medicine and environmental health. 2013; 26(3): 430–9.
52.
Elseviers MM, Arias-Guillen M, Gorke A, Arens HJ. Sharps injuries amongst healthcare workers: review of incidence, transmissions and costs. Journal of renal care. 2014; 40(3): 150–6.
53.
Fourati S, Feld JJ, Chevaliez S, Luhmann N. Approaches for simplified HCV diagnostic algorithms. Journal of the International AIDS Society. 2018; 21 Suppl 2: e25058.
54.
Piekarska A, Tomasiewicz K, Halota W, Jaroszewicz J, Krygier R, Małkowski P, et al. Searching for the optimal population for hepatitis C virus screening in Poland. Clinical and Experimental Hepatology. 2020; 6(2): 74–6.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.