Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Impact of the oxidative and enzymatic metals in degenerated intervertebral disc disease
1
Department of Spine Orthopedics and Biomechanics, W. Dega University Hospital, University of Medical Sciences,
Poznan, Poland
2
Faculty of Chemistry, Department of Analytical Chemistry, Adam Mickiewicz University Poznan, Poland
3
Provincial Hospital of Alfred Sokołowski, Złotów, Poland
4
Faculty of Chemistry, Department of Analytical and Environmental Chemistry, Adam Mickiewicz University, Poznan,
Poland
5
University of Medical Sciences, Poznan, Poland
Corresponding author
Mikołaj Dąbrowski
Poznan University of Medical Sciences, 28 Czerwca 1956 135/147, 61-545, Poznan, Poland
Poznan University of Medical Sciences, 28 Czerwca 1956 135/147, 61-545, Poznan, Poland
Ann Agric Environ Med. 2021;28(3):491-501
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The degenerative process of the intervertebral disc is a heterogeneous process that may exist in two forms, and involves dominant degenerative changes within the nucleus pulposus and the annulus fibrosus. In degenerative disc disease, the oxidative stress factor can play an important role.
Objective:
The aim of research was to present a new approach to understanding the role of the analyzed elements in the process of degeneration of the intervertebral disc.
Material and methods:
Selected elements from oxidative groups (Fe, Zn, Mo, As, Se), associated with enzymatic processes (Fe, Mo, Se, Zn, Ag, As, Bi), metals (Fe, Zn, Mo, Li) and metalloids (As, Bi) and their content was analyzed depending on the changes in the radiological images of the intervertebral disc. Elemental content analysis was performed by Inductively Coupled Plasma Mass Spectrometry analytical technique.
Results:
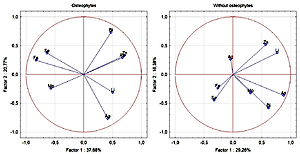
The similarity between Fe and Se has been demonstrated during different stages of the analysis of groups of patients with degenerative disc disease. There was a negative correlation between Li and degenerative disc disease. The results also suggest that Fe and Ag are involved in degenerative changes within the intervertebral disc. A potential relationship between As/Bi and Fe/Mo in the degeneration of the intervertebral disc was demonstrated.
Conclusions:
Only some of the correlations can be explained by the metabolism of morphological elements of the intervertebral disc. The relationships indicate new directions for further studies on the degeneration process of the intervertebral disc. The presented study may reflect metabolic changes in the intervertebral disc and adjacent structures in response to the progressive degenerative process.
The degenerative process of the intervertebral disc is a heterogeneous process that may exist in two forms, and involves dominant degenerative changes within the nucleus pulposus and the annulus fibrosus. In degenerative disc disease, the oxidative stress factor can play an important role.
Objective:
The aim of research was to present a new approach to understanding the role of the analyzed elements in the process of degeneration of the intervertebral disc.
Material and methods:
Selected elements from oxidative groups (Fe, Zn, Mo, As, Se), associated with enzymatic processes (Fe, Mo, Se, Zn, Ag, As, Bi), metals (Fe, Zn, Mo, Li) and metalloids (As, Bi) and their content was analyzed depending on the changes in the radiological images of the intervertebral disc. Elemental content analysis was performed by Inductively Coupled Plasma Mass Spectrometry analytical technique.
Results:
The similarity between Fe and Se has been demonstrated during different stages of the analysis of groups of patients with degenerative disc disease. There was a negative correlation between Li and degenerative disc disease. The results also suggest that Fe and Ag are involved in degenerative changes within the intervertebral disc. A potential relationship between As/Bi and Fe/Mo in the degeneration of the intervertebral disc was demonstrated.
Conclusions:
Only some of the correlations can be explained by the metabolism of morphological elements of the intervertebral disc. The relationships indicate new directions for further studies on the degeneration process of the intervertebral disc. The presented study may reflect metabolic changes in the intervertebral disc and adjacent structures in response to the progressive degenerative process.
ACKNOWLEDGEMENTS
Funding source. National Science Center in Poland
(MINIATURA 1 2017), under grant agreement no. DEC-
2017/01/X/NZ5/00308.
REFERENCES (21)
1.
Binch AL, Cole AA, Breakwell LM, et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther. 2015; 17(1): 370.
2.
Gilbert HTJ, Hodson N, Baird P, Richardson SM, Hoyland JA. Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target. Sci Rep. 2016; 6: 37360.
3.
Wu Y, Cisewski S, Sachs BL, Yao H. Effect of cartilage endplate on cell based disc regeneration: a finite element analysis. Mol Cell Biomech. 2013; 10(2): 159–82.
4.
Le Maitre CL, Richardson SM, Baird P, Freemont AJ, Hoyland JA. Expression of receptors for putative anabolic growth factors in human intervertebral disc: implications for repair and regeneration of the disc. J Pathol. 2005; 207(4): 445–52.
5.
Suzuki S, Fujita N, Hosogane N, et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015; 17(1): 316.
6.
Zioła-Frankowska A, Kubaszewski Ł, Dąbrowski M, et al. The content of the 14 metals in cancellous and cortical bone of the hip joint affected by osteoarthritis. BioMed Res Int. 2015; 2015.
7.
Kubaszewski Ł, Zioła-Frankowska A, Frankowski M, et al. Atomic absorption spectrometry analysis of trace elements in degenerated intervertebral disc tissue. Med Sci Monitor: Int Med J Exp Clin Res. 2014; 20: 2157.
8.
Nowakowski A, Kubaszewski L, Frankowski M, et al. Analysis of trace element in intervertebral disc by atomic absorption spectrometry techniques in degenerative disc disease in the Polish population. Ann Agric Environ Med. 2015; 22(2).
9.
Kubaszewski Ł, Zioła-Frankowska A, Frankowski M, et al. Comparison of trace element concentration in bone and intervertebral disc tissue by atomic absorption spectrometry techniques. J Orthopaedic Surg Res. 2014; 9(1): 99.
10.
Saleem S, Aslam HM, Rehmani MA, Raees A, Alvi AA, Ashraf J. Lumbar disc degenerative disease: disc degeneration symptoms and magnetic resonance image findings. Asian Spine J. 2013; 7(4): 322–34.
11.
Li SY, Cao JL, Shi ZL, et al. Promotion of the articular cartilage proteoglycan degradation by T-2 toxin and selenium protective effect. J Zhejiang Univ Sci B. 2008; 9(1): 22–33.
12.
Yasuda S, Yasuda T, Liu MY, et al. Sulfation of chlorotyrosine and nitrotyrosine by human lung endothelial and epithelial cells: role of the human SULT1A3. Toxicol Appl Pharmacol. 2011; 251(2): 104–9.
13.
Lu M, Cao J, Liu F, et al. The effects of mycotoxins and selenium deficiency on tissue-engineered cartilage. Cells Tissues Organs. 2012; 196(3): 241–50.
14.
Medeiros DM. Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: A review. Exp Biol Med. 2016; 241(12): 1316–22.
15.
Toxqui L, Vaquero M. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015; 7(4): 2324–44.
16.
Min Z, Zhao W, Zhong N, et al. Abnormality of epiphyseal plate induced by selenium deficiency diet in two generation DA rats. APMIS. 2015; 123(8): 697–705.
17.
Kwan KH, Yeung KW, Liu X, et al. Silver nanoparticles alter proteoglycan expression in the promotion of tendon repair. Nanomedicine: Nanotechnol Biol Med. 2014; 10(7): 1375–83.
18.
Hays AM, Lantz RC, Rodgers LS, et al. Arsenic-induced decreases in the vascular matrix. Toxicol Pathol. 2008; 36(6): 805–17.
19.
Rade M, Määttä JH, Freidin MB, Airaksinen O, Karppinen J, Williams FM. Vertebral Endplate Defect as Initiating Factor in Intervertebral Disc Degeneration. Spine. 2018; 43(6): 412–9.
20.
Fujiwara Y, Yamamoto C, Inagaki T, Satoh M, Kaji T. Bismuth protects against arsenite-induced inhibition of proteoglycan synthesis in cultured vascular endothelial cells. J Toxicol Sci. 2012; 37(4): 837–43.
21.
Mendel RR. Metabolism of molybdenum. Met Ions Life Sci. 2013; 12: 503–28.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.