Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
How to get rid of ticks – a mini-review on tick control strategies in parks, gardens, and other human-related environments
1
Department of Eco-Epidemiology of Parasitic Diseases, University of Warsaw, Poland
Corresponding author
Dagmara Wężyk
Address for correspondence: Dagmara Wężyk, Department of Eco-Epidemiology of Parasitic Diseases, Medical University, Warsaw, Poland
Address for correspondence: Dagmara Wężyk, Department of Eco-Epidemiology of Parasitic Diseases, Medical University, Warsaw, Poland
Ann Agric Environ Med. 2025;32(1):1-8
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
In recent years, the geographical range of many tick species has expanded significantly, increasing the threat of emerging tick-borne diseases. Th aim of this review is to assess the current state of tick control in the environment, highlighting the limitations of existing methods and the need to develop new approaches.
Review methods:
The literature was systematically reviewed using such databases as Google Scholar, PubMed, and ResearchGate. Key word searches focused on tick control and prevention of tick-borne diseases in environments. Evaluation criteria included efficacy and feasibility of various tick control measures.
Brief description of the state of knowledge:
Biological control of ticks in the environment relies on entomopathogenic fungi, particularlythe Metarhizium species, delivered as granules. Synthetic acaricides, including pyrethroids and organophosphates, are widely used, with liquid formulations proving more effective than granules. Innovative approaches, such as the TickBot robot and devices targeting reservoir hosts such as deer, show promise. Future directions include the development of vaccines targeting tick antigens, and translational biotechnological strategies for tick population control.
Summary:
The prevention of tick-borne diseases involves various control methods, such as the use of acaricides, fungi, pheromones and innovative approaches, such as bait tubes and boxes. Each method has its own set of pros and cons, emphasizing the need for an integrated and strategic approach. While innovative methods, including vaccines and molecular approaches, show promise, further research and testing in natural environments are necessary to confirm their effectiveness. Achieving long-lasting and comprehensive tick control remains a challenging task in promoting public health
In recent years, the geographical range of many tick species has expanded significantly, increasing the threat of emerging tick-borne diseases. Th aim of this review is to assess the current state of tick control in the environment, highlighting the limitations of existing methods and the need to develop new approaches.
Review methods:
The literature was systematically reviewed using such databases as Google Scholar, PubMed, and ResearchGate. Key word searches focused on tick control and prevention of tick-borne diseases in environments. Evaluation criteria included efficacy and feasibility of various tick control measures.
Brief description of the state of knowledge:
Biological control of ticks in the environment relies on entomopathogenic fungi, particularlythe Metarhizium species, delivered as granules. Synthetic acaricides, including pyrethroids and organophosphates, are widely used, with liquid formulations proving more effective than granules. Innovative approaches, such as the TickBot robot and devices targeting reservoir hosts such as deer, show promise. Future directions include the development of vaccines targeting tick antigens, and translational biotechnological strategies for tick population control.
Summary:
The prevention of tick-borne diseases involves various control methods, such as the use of acaricides, fungi, pheromones and innovative approaches, such as bait tubes and boxes. Each method has its own set of pros and cons, emphasizing the need for an integrated and strategic approach. While innovative methods, including vaccines and molecular approaches, show promise, further research and testing in natural environments are necessary to confirm their effectiveness. Achieving long-lasting and comprehensive tick control remains a challenging task in promoting public health
REFERENCES (65)
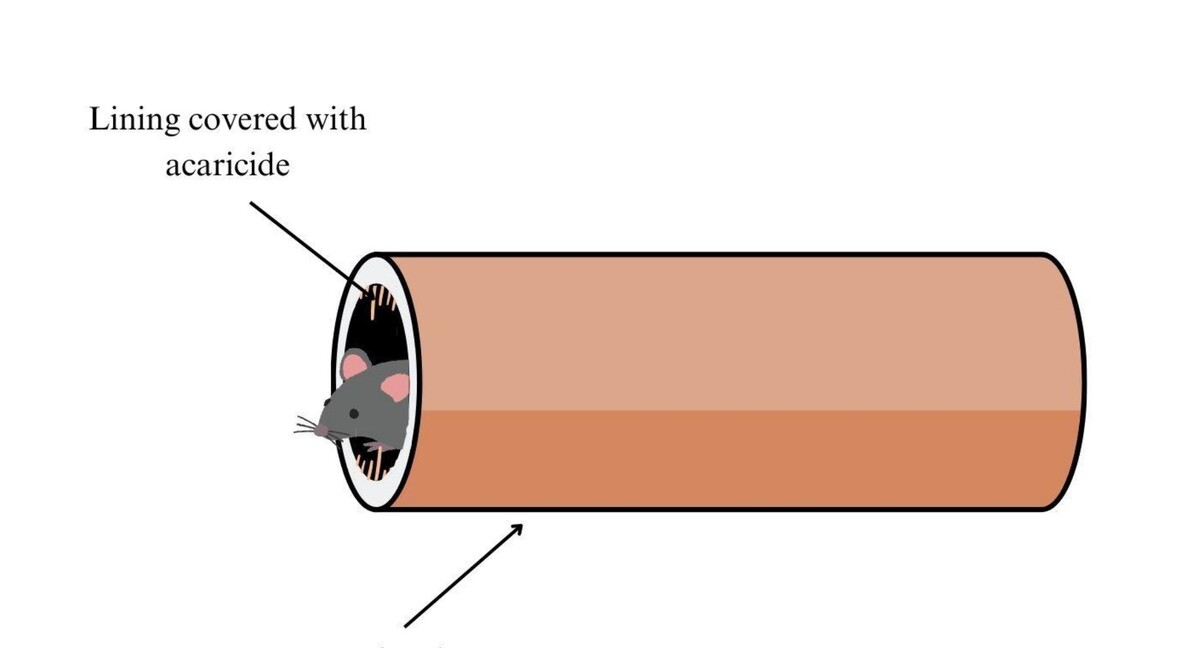
1.
Guglielmone AA, Robbins RG, Apanaskevich DA, et al. The hard ticks of the world (Acari: Ixodida: Ixodidae). Springer, Switzerland; 2014. p. 1–7.
2.
Rochlin I, Toledo A. Emerging tick-borne pathogens of public health importance: a mini-review. J Med Microbiol. 2020;69(6):781–791. doi: 10.1099/jmm.0.001206.
3.
Camicas JL, Hervy JP, Adam F, et al. The ticks of the world (Acarida, Ixodida): nomenclature, described stages, hosts, distribution. Paris: ORSTOM; 1998. p. 223.
4.
Batool M, Nasir S, Rafique A, et al. Prevalence of tick infestation in farm animals from Punjab, Pakistan. Pak Vet J. 2019;39(3):406–410. http://dx.doi.org/10.29261/pak....
5.
Springer A, Glass A, Topp AK, et al. Zoonotic tick-borne pathogens in temperate and cold regions of Europe—A review on the prevalence in domestic animals. Front Vet Sci. 2020;7:604910. doi:10.3389/ fvets.2020.604910.
6.
Boulanger N, Boyer P, Talagrand-Reboul E, et al. Ticks and tick-borne diseases. Med Mal Infec. 2019;49(2):87–97. doi:10.1016 j.medmal.2019.01.007.
7.
Githaka NW, Kanduma EG, Wieland B, et al. Acaricide resistance in livestock ticks infesting cattle in Africa: Current status and potential mitigation strategies. Curr Res Parasitol Vector Borne Dis. 2022;100090. 10.1016/j.crpvbd.2022.100090.
8.
Nath S, Mandal S, Pal S, et al. Impact and management of acaricide resistance-pertaining to sustainable control of ticks. Int J Livest Res. 2018;8(10):46–60. doi:10.5455/ijlr.20180402121612.
9.
Richardson M, Khouja C, Sutcliffe K. Interventions to prevent Lyme disease in humans: A systematic review. Prev Med Rep. 2018;13:16–22. doi:10.1016/j.pmedr.2018.11.004.
10.
Gonzaga BCF, Barrozo MM, Coutinho AL. Essential oils and isolated compounds for tick control: advances beyond the laboratory. Parasit Vectors. 2023;16:415. https://doi.org/10.1186/s13071....
11.
Robinson W. Urban entomology: insect and mite pests in the human environment. In: Robinson WH. Urban Entomology. London: Chapman and Hall; 1996. p. 285–320.
12.
Jamil M, Latif N, Gul J, et al. A review: An insight into the potential of biological control of ticks in domestic and wild animals. AJLS. 2022;5(2):51–67. doi:10.34091/AJLS.5.2.6.
13.
Ebani VV, Mancianti F. Entomopathogenic fungi and bacteria in a veterinary perspective. Biology. 2021;10:479. https://doi.org/10.3390/ biology10060479.
14.
Deveau A, Bonito G, Uehling J. Bacterial–fungal interactions: ecology, mechanisms and challenges. FEMS Microbiol Rev. 2018;42(3):335–352. doi:10.1093/femsre/fuy008. PMID:29471481.
15.
Mascarin G, Lopes R, Delaliber I, et al. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J Invertebr Pathol. 2019;165:46–53. https://doi. org/10.1016/j.jip.2018.01.001.
16.
Sullivan CF, Parker BL, Skinner M. A review of commercial Metarhizium-and Beauveria-based biopesticides for the biological control of ticks in the USA. Insects. 2022;13(3):260. doi:10.3390/ insects13030260.
17.
Patil S, Sarraf G, Kharkwal AC. Panorama of Metarhizium: Host interaction and its uses in biocontrol and plant growth promotion. In: Shrivastava N, Mahajan S, Varma A, editors. Symbiotic Soil Mikroorganizm: Soil Biology. Springer; 2021. p. 289–318. https://doi. org/10.1007/978-3-030-51916-2_18.
18.
Ghazanfar MU, Raza M, Raza W, et al. Trichoderma as potential biocontrol agent, its exploitation in agriculture: a review. Plant Protection. 2018;2(3):109–135.
19.
Mastropaolo M, Mangold AJ, Guglielmone AA, et al. Non-parasitic life cycle of the cattle tick Rhipicephalus (Boophilus) microplus in Panicum maximum pastures in northern Argentina. Res Vet Sci. 2017;115:138–145. doi:10.1016/j.rvsc.2017.03.009.
20.
Marciano AF, Mascarin F, Franco R, et al. Innovative granular formulation of Metarhizium robertsii microsclerotia and blastospores for cattle tick control. Sci Rep. 2021;11:4972. https://doi.org/10.1038/ s41598-021-84142-8.
21.
Bernardo CC, Barreto L, Silva C, et al. Conidia and blastospores of Metarhizium spp. and Beauveria bassiana s.l.: their development during the infection process and virulence against the tick Rhipicephalus microplus. Ticks Tick-Borne Dis. 2018;9(5):1334–1342.
22.
Camargo MG, Golo PS, Angelo IC, et al. Effect of oil-based formulations of acaripathogenic fungi to control Rhipicephalus microplus ticks under laboratory conditions. Vet Parasitol. 2012;188:140–147. doi:10.1016/j. ttbdis.2018.06.001.
23.
Fernández-Salas A, Alonso-Diaz M, Alonso-Morales R, et al. Acaricidal activity of Metarhizium anisopliae isolated from paddocks in the Mexican tropics against two populations of the cattle tick Rhipicephalus microplus. Med Vet Entomol. 2017;31(1):36–43. doi:10.1111/mve.12203.
24.
Eisen L, Dolan MC. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J Med Entomol. 2016;53(5):1063–1092. doi:10.1093/ jme/tjw103.
25.
Bron GM, Lee X, Paskewitz SM. Do-it-yourself tick control: granular gamma-cyhalothrin reduces Ixodes scapularis (Acari: Ixodidae) nymphs in residential backyards. J Med Entomol. 2021;58(2):749–755. https://doi.org/10.1093/jme/tj....
26.
Schulze TL, Jordan R. Early season applications of bifenthrin suppress host-seeking Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. J Med Entomol. 2019;57(3):807–814. doi:10.1093/ jme/tjz202.
27.
Jordan RA, Schulze TL, Eisen L, et al. Ability of three general-use pesticides to suppress nymphal Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae). J Am Mosq Control Assoc. 2017;33910:50–55. doi:10.2987/16-6610.1.
28.
Schulze TL, Jordan R, Hung W, et al. Efficacy of granular deltamethrin against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidade) nymphs. J Med Entomol. 2001;38(2):344–346. doi:10.1603/0022-2585-38.2.344.
29.
Schulze TL, Taylor G, Jordan R, et al. Effectiveness of selected granular acaricide formulations in suppressing populations of Ixodes dammini (Acari: Ixodidae): short-term control of nymphs and larvae. J Med Entomol. 1991;28(5):624–629. doi:10.1093 jmedent/28.5.624.
30.
Solberg V, Neidhardt K, Sardelis M, et al. Field evaluation of two formulations of cyfluthrin for control of Ixodes dammini and Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1992;29(2):634–638. doi:10.1093/jmedent/29.4.634.
31.
Smith Jr, Jackson P. Effects of insecticidal placement on non-target arthropods in the peanut ecosystem. Peanut Sci. 1975;2(2):87–90. https://doi.org/10.3146/i0095-....
32.
van Wieren SE, Brak MA, Lahr J. Effectiveness and environmental hazards of acaricides applied to large mammals for tick control. In Ecology and prevention of Lyme borreliosis. Nederlands: Wageningen Academic Publishers; 2016. p. 75–89. https://doi.org/10.3920/978-90....
33.
Agwunobi DO, Yu Z, Liu J. A retrospective review on ixodid tick resistance against synthetic acaricides: Implications and perspectives for future resistance prevention and mitigation. Pestic Bioche Physiol. 2021;173:104776. doi:10.1016 j.pestbp.2021.104776.
34.
Sonenshine DE. Tick pheromones and their use in tick control. Ann Rev Entomol. 2006;51:557–580. doi:10.1146/annurev.ento.51.110104.151150.
35.
Allan S, Sonenshine D, Burridge M, et al. Tick pheromones and uses thereof. U.S.Patent No. 6,331,297, B1, 2001.
36.
Gaff HD, White A, Leas K, et al. TickBot: a novel robotic device for controlling tick populations in the natural environment. Ticks Tick-Borne Dis. 2016;6(2):146–151. doi:10.1016/j.ttbdis.2014.11.004.
37.
Stafford KC. III. Tick Management Handbook: An integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease. Connecticut Agricultural Experiment Station, Bulletin 1010; 2007. p. 78.
38.
Heylen D, Lasters R, Adriaensen F, et al. Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area. Sci Total Environ. 2019;670:941– 949. doi:10.1016/j.scitotenv.2019.03.235.
39.
Goethert HK, Telford III, SR. Limited capacity of deer to serve as zooprophylactic hosts for Borrelia burgdorferi in the Northeastern United States. Appl Environ Microbiol. 2022;88(6):22–42. doi:10.1128/ aem.00042-22.
40.
Telford III, SR. Deer reduction is a cornerstone of integrated deer tick management. J Integr Pest Manag. 2017;8(1):25. https://doi.org/10.1093/ jipm/pmx024.
41.
Sonenshine DE, Allan S, Norval R, et al. A self-medicating applicator for control of ticks on deer. Med Vet Entomol. 1996;10(2):149–154. doi: 10.1111/j.1365–2915.1996.tb00721.x.
42.
Pound JM, Miller J, George J. Efficacy of amitraz applied to white-tailed deer by the ‘4-poster’ topical treatment device in controlling free-living lone star ticks (Acari: Ixodidae). J Med Entomol. 2000a;37(6):878–884. doi:10.1603/0022-2585-37.6.878.
43.
Pound JM, Miller J, George J, et al. The ‘4-Poster’ passive tropical treatment device to apply acaricide for controlling ticks (Acari: Ixodidae) feeding on white-tailed deer. J Med Entomol. 2000b;37(4):588–594. doi:10.1603/0022-2585-37.4.588.
44.
Solberg V, Miller J, Hadfield T, et al. Control of Ixodes scapularis (Acari: Ixodidae) with topical self-application of permethrin by whitetailed deer inhabiting NASA, Beltsville, Maryland. J Vector Ecol. 2003;28(1):117–134.
45.
Poché D, Wagner D, Green K, et al. Development of a low-dose fipronil deer feed: evaluation of efficacy against two medically important tick species parasitizing white-tailed deer (Odocoileus virginianus) under pen conditions. Parasit Vectors. 2003;16:1–20. https://doi.org/10.1186/ s13071-023-05689-1.
46.
Kartman L. An insecticide-bait-box method for the control of sylvatic plague vectors. Epidemiol Infect. 1958;56(4):455–465. doi:10.1017/ S0022172400037967.
47.
Dolan MC, Maupin G, Schneider B, et al. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J Med Entomol. 2004;41(6):1043–1054. doi:10.1603/0022-2585-41.6.1043.
48.
Schulze TL, Jordan R, Williams M, et al. Evaluation of the SELECT tick control system (TCS), a host-targeted bait box, to reduce exposure to Ixodes scapularis (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey. J Med Entomol. 2017;54(4):1019–1024. doi:10.1093/ jme/tjx044.
49.
Hinckley AF, Niesobecki SA, Connally NP. Prevention of Lyme and other tickborne diseases using a rodent-targeted approach: A randomized controlled trial in Connecticut. Zoonoses Public Health. 2021;68(6):578–587. doi:10.1111/zph.12844.
50.
Lane R, Casher L, Peavey C, et al. A better tick-control trap: modified bait tube controls disease-carrying ticks and fleas. California Agriculture. 1998;52:43–47.
51.
Gage K, Maupin E, Montenieri J, et al. Flea (Siphonaptera: Ceratophyllidae, Hystrichopsyllidae) and tick (Acarina: Ixodidae) control on wood rats using host-targeted liquid permethrin in bait tubes. J Med Entomol. 1997;34(1):46–51. doi:10.1093 jmedent/34.1.46.
52.
Brown JE, Miller TM, Machtinger ET. Tick tubes reduce blacklegged tick burdens on white-footed mice in Pennsylvania, USA. J Appl Entomol. 2020;144(6):542–545. https://doi.org/10.1111/jen.12....
53.
Sajid A, Matias J, Arora G, et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent. Sci Transl Med. 2021;13(620):eabj9827. doi:10.1126/scitranslmed.abj9827.
54.
Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi:10.1016/j. jconrel.2015.08.007.
55.
Valle MR, Guerrero FD. Anti-tick vaccines in the omics era. Front Biosci (Elite Ed). 2018;10(1):122–136. doi:10.2741/e812.
56.
Almazan C, Tipacamu GA, Rodriguez S, et al. Immunological control of ticks and tick-borne diseases that impact cattle health and production. Front Biosci (Landmark Ed). 2018;23(8):1535–1551. doi:10.2741/4659.
57.
de la Fuente J. Translational biotechnology for the control of ticks and tick-borne diseases. Ticks Tick Borne Dis. 2021;12(5):101738. doi:10.1016/j.ttbdis.2021.101738.
58.
Díaz-Sánchez S, Estrada-Peña A, Cabezas-Cruz A, et al. Evolutionary insights into the tick hologenome. Trends Parasitol. 2019;35(9):725–737. doi:10.1016/j.pt.2019.06.014.
59.
Artigas-Jerónimo S, Villar M, Cabezas-Cruz A, et al. Tick Importin-α is implicated in the interactome and regulome of the cofactor Subolesin. Pathogens. 2021;10(4):457. doi:10.3390/pathogens10040457.
60.
Suppan J, Engel B, Marchetti-Deschmann M, et al. Tick attachment cement–reviewing the mysteries of a biological skin plug system. Biol Rev Camb Philos Soc. 2018;93(2):1056–1076. doi:10.1111/brv.12384.
61.
Cote J, Ada E, Hochberg R. Elemental enrichment of the exoskeleton in three species of tick (Arachnida: Ixodidae). J Parasitol. 2020;106(6):742–754. doi:10.1645/20-95.
62.
Sharma A, Pham MN, Reyes JB, et al. Cas9-mediated gene editing in the black-legged tick, Ixodes scapularis, by embryo injection and ReMOT Control. I Sci. 2022;25(3):103781. doi:10.1016/j.isci.2022.103781.
63.
Beys-da-Silva WO, Rosa RL, Berger M, et al. Updating the application of Metarhizium anisopliae to control cattle tick Rhipicephalus microplus (Acari: Ixodidae). Exp Parasitol. 2020;208:107812. doi:10.1016/j. exppara.2019.107812.
64.
NASA Langley Research Center. The ‘TickBot’ Takes the Bite out of Bugs. The ‘TickBot’ Takes the Bite out of Bugs (youtube.com) (access: 2018.05.29).
65.
Staten Island Borough President Vito J. Fossella. Deer four-poster feeding station eliminating ticks and Lyme disease. https://www. youtube.com/watch?v=rVbByLywpQw (access: 2019.07.15).
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.