Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
How 5-ALA enlightens neurosurgery – results of a single centre study on high-grade gliomas
1
Department of Neurosurgery and Paediatric Neurosurgery, Medical University, Lublin, Poland
Corresponding author
Natalia Lehman
Department of Neurosurgery and Paediatric Neurosurgery, Medical University of Lublin, Jaczewskiego, 20-093, Lublin, Poland
Department of Neurosurgery and Paediatric Neurosurgery, Medical University of Lublin, Jaczewskiego, 20-093, Lublin, Poland
Ann Agric Environ Med. 2025;32(1):133-141
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
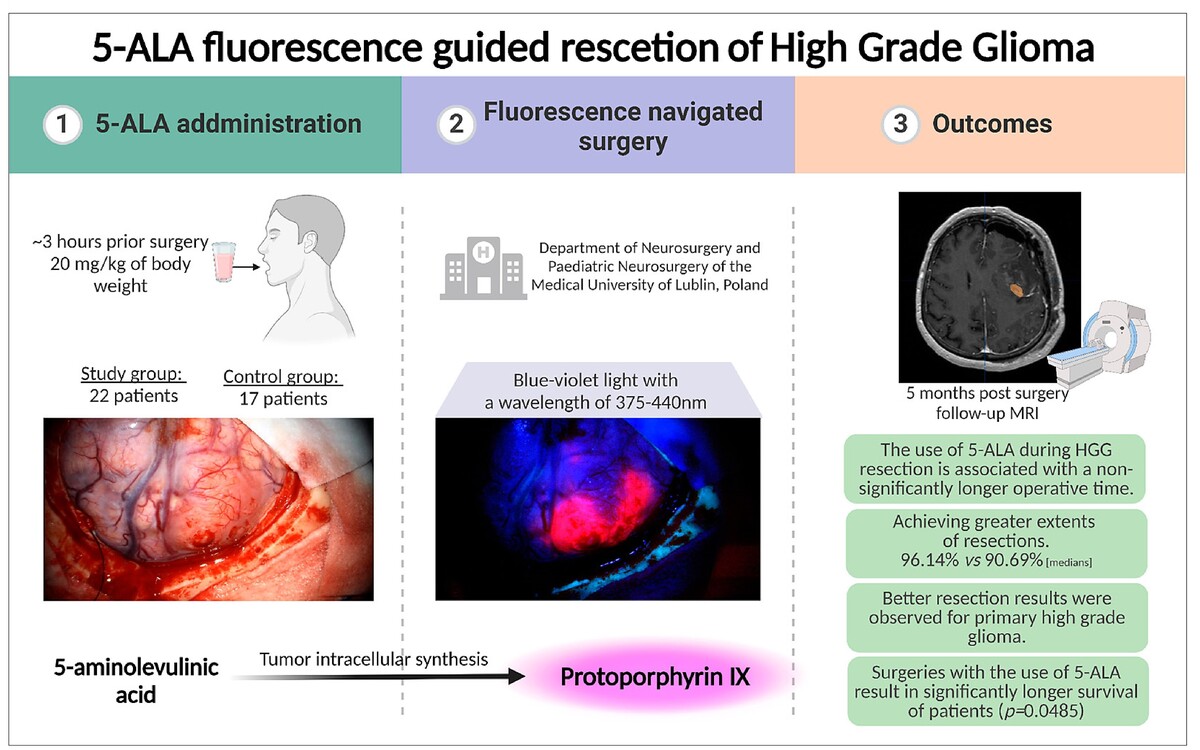
This study aims to assess the effectiveness of the use of intraoperative fluorescence with 5-ALA on the scope on the resection and the results of the treatment of patients. Despite the continuous development of new oncological treatments, surgical resection remains the basis for treating high-grade gliomas. For this reason, methods are introduced to facilitate the removal of the tumour in a maximally complete and safe manner for the patient.
Material and methods:
The effects were examined of intraoperative fluorescence using 5-aminolevulinic acid (5-ALA). The volumetric extent of resection and the outcome of 39 cases of patients with high-grade gliomas operated on using intraoperative fluorescence were compared with 5-ALA and patients undergoing resection using a white-light microscope.
Results:
The use of 5-ALA during the procedure increased the extent of resection compared to procedures under white light: – 96.14% vs. 90.69%. Interestingly, when focused on recurrent high-grade gliomas, better results were observed for the white-light group. There was also an improvement in the overall survival (OS) of patients operated on with 5-ALA (p=0.0485). OS for the study group was 9.97 months compared to 6.40 months for the control group.
Conclusions:
Based on the observations, the use of 5-ALA in surgery for high-grade gliomas allows for an increase in the extent of resection performed and an improvement in patient prognosis. Moreover, the use of 5-ALA shows better results for primary tumours when compared to recurrent ones (p<0.0001 for primary gliomas).
This study aims to assess the effectiveness of the use of intraoperative fluorescence with 5-ALA on the scope on the resection and the results of the treatment of patients. Despite the continuous development of new oncological treatments, surgical resection remains the basis for treating high-grade gliomas. For this reason, methods are introduced to facilitate the removal of the tumour in a maximally complete and safe manner for the patient.
Material and methods:
The effects were examined of intraoperative fluorescence using 5-aminolevulinic acid (5-ALA). The volumetric extent of resection and the outcome of 39 cases of patients with high-grade gliomas operated on using intraoperative fluorescence were compared with 5-ALA and patients undergoing resection using a white-light microscope.
Results:
The use of 5-ALA during the procedure increased the extent of resection compared to procedures under white light: – 96.14% vs. 90.69%. Interestingly, when focused on recurrent high-grade gliomas, better results were observed for the white-light group. There was also an improvement in the overall survival (OS) of patients operated on with 5-ALA (p=0.0485). OS for the study group was 9.97 months compared to 6.40 months for the control group.
Conclusions:
Based on the observations, the use of 5-ALA in surgery for high-grade gliomas allows for an increase in the extent of resection performed and an improvement in patient prognosis. Moreover, the use of 5-ALA shows better results for primary tumours when compared to recurrent ones (p<0.0001 for primary gliomas).
REFERENCES (25)
1.
Legmouz M, Ouahabi AE, Boulbaroud S, Azzaoui FZ. BRAIN TUMOURS AMONG MOROCCAN ADOLESCENTS IN THE REGION OF RABAT-SALE-KENITRA, MOROCCO. Acta Neuropsychol. 2022;20(3):345–353. doi:10.5604/01.3001.0016.0760.
2.
Obrador E, Moreno-Murciano P, Oriol-Caballo M, et al. Glioblastoma Therapy: Past, Present and Future. International Journal of Molecular Sciences. 2024;25(5):2529. doi:10.3390/ijms25052529.
3.
Della Pepa GM, Ius T, La Rocca G, et al. 5-Aminolevulinic Acid and Contrast-Enhanced Ultrasound: The Combination of the Two Techniques to Optimize the Extent of Resection in Glioblastoma Surgery. Neurosurgery. 2020;86(6):E529-E540. doi:10.1093/neuros/nyaa037.
4.
Mazurek M, Kulesza B, Stoma F, Osuchowski J, Mańdziuk S, Rola R. Characteristics of Fluorescent Intraoperative Dyes Helpful in Gross Total Resection of High-Grade Gliomas-A Systematic Review. Diagnostics (Basel). 2020;10(12):1100. doi:10.3390/diagnostics10121100.
5.
Eatz TA, Eichberg DG, Lu VM, Di L, Komotar RJ, Ivan ME. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol. 2022;156(2):233–256. doi:10.1007/s11060–021–03901–9.
6.
Kim SK, Choi SH, Kim YH, Park CK. Impact of fluorescence-guided surgery on the improvement of clinical outcomes in glioblastoma patients. Neurooncol Pract. 2014;1(3):81–85. doi:10.1093/nop/npu011.
7.
Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi:10.3171/jns.2001.95.2.0190.
8.
Suchorska B, Weller M, Tabatabai G, et al. Complete resection of contrast-enhancing tumour volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18(4):549–556. doi:10.1093/neuonc/nov326.
9.
Ahrens LC, Krabbenhøft MG, Hansen RW, et al. Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers (Basel). 2022;14(3):617. doi:10.3390/cancers14030617.
10.
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003–1013. doi:10.3171/jns.2000.93.6.1003.
11.
Kim S, Kim JE, Kim YH, et al. Glutaminase 2 expression is associated with regional heterogeneity of 5-aminolevulinic acid fluorescence in glioblastoma. Sci Rep. 2017;7(1):12221. doi:10.1038/s41598–017–12557–3.
12.
Ng WP, Liew BS, Idris Z, Rosman AK. Fluorescence-Guided versus Conventional Surgical Resection of High Grade Glioma: A Single-Centre, 7-Year, Comparative Effectiveness Study. Malays J Med Sci. 2017;24(2):78–86. doi:10.21315/mjms2017.24.2.10.
13.
Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi:10.1016/S1470–2045(06)70665–9.
14.
Picart T, Armoiry X, Berthiller J, et al. Is fluorescence-guided surgery with 5-ala in eloquent areas for malignant gliomas a reasonable and useful technique? Neurochirurgie. 2017;63(3):189–196. doi:10.1016/j.neuchi.2016.12.005.
15.
Belykh E, Miller EJ, Patel AA, et al. Optical Characterization of Neurosurgical Operating Microscopes: Quantitative Fluorescence and Assessment of PpIX Photobleaching. Sci Rep. 2018;8(1):12543. doi:10.1038/s41598–018–30247–6.
16.
Ohba S, Murayama K, Kuwahara K, et al. The Correlation of Fluorescence of Protoporphyrinogen IX and Status of Isocitrate Dehydrogenase in Gliomas. Neurosurgery. 2020;87(2):408–417. doi:10.1093/neuros/nyz524.
17.
Jaber M, Wölfer J, Ewelt C, et al. The Value of 5-Aminolevulinic Acid in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, Magnetic Resonance Imaging, 18F-Fluoroethyl Tyrosine Positron Emission Tomography, and Tumour Molecular Factors. Neurosurgery. 2016;78(3):401–411. doi:10.1227/NEU.0000000000001020.
18.
Aknin LB, Neve JED, Dunn EW, et al. THE NEUROLOGICAL CONSEQUENCES OF CONTRACTING COVID-19. Acta Neuropsychologica. 2021;19(3):301–305. doi:10.5604/01.3001.0014.9953.
19.
Sakurai Y, Ngwe Tun MM, Kurosaki Y, et al. 5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro. Biochem Biophys Res Commun. 2021;545:203–207. doi:10.1016/j.bbrc.2021.01.091.
20.
Ricciardi L, Sturiale CL, Scerrati A, et al. 5-Aminolevulinic Acid False-Positive Rates in Newly Diagnosed and Recurrent Glioblastoma: Do Pseudoprogression and Radionecrosis Play a Role? A Meta-Analysis. Front Oncol. 2022;12:848036. doi:10.3389/fonc.2022.848036.
21.
Beika M, Harada Y, Minamikawa T, et al. Accumulation of Uroporphyrin I in Necrotic Tissues of Squamous Cell Carcinoma after Administration of 5-Aminolevulinic Acid. Int J Mol Sci. 2021;22(18):10121. doi:10.3390/ijms221810121.
22.
Wadiura LI, Mischkulnig M, Hosmann A, et al. Influence of Corticosteroids and Antiepileptic Drugs on Visible 5-Aminolevulinic Acid Fluorescence in a Series of Initially Suspected Low-Grade Gliomas Including World Health Organization Grade II, III, and IV Gliomas. World Neurosurg. 2020;137:e437-e446. doi:10.1016/j.wneu.2020.01.243.
23.
Teng L, Nakada M, Zhao SG, et al. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer. 2011;104(5):798–807. doi:10.1038/bjc.2011.12.
24.
Slof J, Díez Valle R, Galván J. Cost-effectiveness of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery. Neurologia. 2015;30(3):163–168. doi:10.1016/j.nrl.2013.11.002.
25.
Esteves S, Alves M, Castel-Branco M, Stummer W. A pilot cost-effectiveness analysis of treatments in newly diagnosed high-grade gliomas: the example of 5-aminolevulinic Acid compared with white-light surgery. Neurosurgery. 2015;76(5):552–562; discussion 562. doi:10.1227/NEU.0000000000000673.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.