Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
First metagenomic report of Borrelia americana and Borrelia carolinensis in Poland – a preliminary study
1
Department of Infectious Diseases and Neuroinfections, Medical University of Bialystok, Poland
2
Department of Microbiology, University of Bialystok, Poland
Corresponding author
Anna Moniuszko-Malinowska
Klinika Chorób Zakaźnych i Neuroinfekcji Uniwersytetu Medycznego w Białymstoku, Ul. Żurawia 14,, 15-540, Białystok, Poland
Klinika Chorób Zakaźnych i Neuroinfekcji Uniwersytetu Medycznego w Białymstoku, Ul. Żurawia 14,, 15-540, Białystok, Poland
Ann Agric Environ Med. 2021;28(1):49-55
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Ixodes ricinus (I. ricinus) and Dermacentor reticulatus (D. reticulatus) are the most common ticks in Poland. These ticks contain many bacteria, which compose a microbiome with potential impact on humans. The aim of the study was to discover the microbiome of ticks in Poland.
Material and methods:
Ticks were collected in The Protected Landscape Area of the Bug and Nurzec Valley, Poland, in 2016–2018 by flagging. They were cleaned in 70% ethanol and damaged in mortar with PBS (without Ca2+ and Mg2+ ions). DNA was extracted from the homogenates with spin columns kits, and used as a matrix in end-point PCR for bacterial 16S rRNA fragments amplifications, and further for next generation sequencing (NGS) by ILLUMINA.
Results:
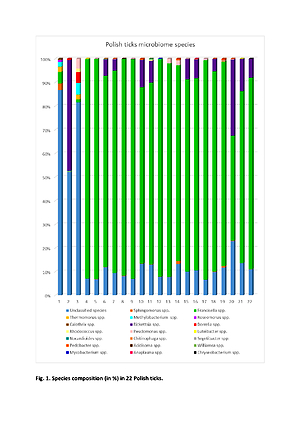
In 22 ticks (3 I. ricinus and 19 D. reticulatus) 38 microorganisms were detected. The most common were Francisella hispaniensis and Francisella novicida. In 17 ticks, Sphingomonas oligophenolica, and in 12 Rickettsia aeshlimanii were found. In 2, I. ricinus specific DNA of Borrelia americana and Borrelia carolinensis were found. In one female, D. reticulatus Anaplasma phagocytophilum and Anaplasma centrale were found. Pseudomonas lutea and Ps. moraviensis were detected in 9 and 8 ticks, respectively.
Conclusions:
Polish ticks microbiome contains not only well-known tick-borne pathogens, but also other pathogenic microorganisms. For the first time in Poland, Borrelia americana and Borrelia carolinensis in I. ricinus collected from the environment were detected. The dominant pathogenic microorganisms for humans were Francisella spp. and Rickettsia spp., and non-pathogenic – Sphingomonas oligophenolica. Knowledge of a tick microbiome might be useful in tick-borne biocontrol and tick-borne diseases prevention.
Ixodes ricinus (I. ricinus) and Dermacentor reticulatus (D. reticulatus) are the most common ticks in Poland. These ticks contain many bacteria, which compose a microbiome with potential impact on humans. The aim of the study was to discover the microbiome of ticks in Poland.
Material and methods:
Ticks were collected in The Protected Landscape Area of the Bug and Nurzec Valley, Poland, in 2016–2018 by flagging. They were cleaned in 70% ethanol and damaged in mortar with PBS (without Ca2+ and Mg2+ ions). DNA was extracted from the homogenates with spin columns kits, and used as a matrix in end-point PCR for bacterial 16S rRNA fragments amplifications, and further for next generation sequencing (NGS) by ILLUMINA.
Results:
In 22 ticks (3 I. ricinus and 19 D. reticulatus) 38 microorganisms were detected. The most common were Francisella hispaniensis and Francisella novicida. In 17 ticks, Sphingomonas oligophenolica, and in 12 Rickettsia aeshlimanii were found. In 2, I. ricinus specific DNA of Borrelia americana and Borrelia carolinensis were found. In one female, D. reticulatus Anaplasma phagocytophilum and Anaplasma centrale were found. Pseudomonas lutea and Ps. moraviensis were detected in 9 and 8 ticks, respectively.
Conclusions:
Polish ticks microbiome contains not only well-known tick-borne pathogens, but also other pathogenic microorganisms. For the first time in Poland, Borrelia americana and Borrelia carolinensis in I. ricinus collected from the environment were detected. The dominant pathogenic microorganisms for humans were Francisella spp. and Rickettsia spp., and non-pathogenic – Sphingomonas oligophenolica. Knowledge of a tick microbiome might be useful in tick-borne biocontrol and tick-borne diseases prevention.
REFERENCES (36)
1.
De la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, Estrada-Peña A, et al. Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front Cell Infect Microbiol. 2017; 7: 114.
2.
Vayssier-Taussat M, Kazimirova M, Hubalek Z, Hornok S, Farkas R, Cosson JF, et al. Emerging horizon for tick-borne pathogens: from the “one pathogen-one disease” vision to the pathobiom paradigm. Future Microbiol. 2015; 10(12): 2033–2043.
3.
Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, Tomsho LP, Drautz DI, Rizzoli A, Schuster SC. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLOS One. 2011; 6(10): e25604.
4.
Bartosik K, Wiśniowski Ł, Buczek A. Abundance and seasonal activity of adult Dermacentor reticulatus (Acari: Amblyommidae) in eastern Poland in relation to meteorological conditions and the photoperiod. Ann Agric Environ Med. 2011; 18(2): 340–344.
5.
Buczek A, Bartosik K, Zając Z, Stanko M. Host-feeding behavior of Dermacentor reticulatus and Dermacentor marginatus in mono-specific and inter-specific infestations. Parasit Vectors. 2015; 8:470–478.
6.
Karbowiak G. The occurrence of the Dermacentor reticulatus tick – its expansion to new areas and possible causes. Ann Parasitol. 2014; 60(1): 37–47.
7.
Földvári G, Rigó K, Lakos A. Transmission of Rickettsia slovaca and Rickettsia raoultii by male Dermacentor marginatus and Dermacentor reticulatus ticks to humans. Diagnostic Microbiol Infect Dis. 2013; 76: 387–389.
8.
Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S. Geographical distribution of Deracentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016; 7: 224–233.
9.
Greay TL, Gofton AW, Paparini A, Ryan UM, Oskam CL, Irwin PJ. Recent insights into the ticks microbiome gained through next-generation sequencing. Parasit Vectors. 2018; 11: 12.
10.
Estrada-Peña A, Cabezas-Cruz A, Pollet T, Vayssier-Taussat M and Cosson J-F. High troughput sequencing and network analysis disentangle the microbial communities of ticks and host within and between an ecosystems. Front Cell Infect Microbiol. 2018; 8: 236.
11.
Moniuszko A, Dunaj J, Święcicka I, Zambrowski G, Chmielewska-Badora J, Żukiewicz-Sobczak W, et al. Co-infections with Borrelia species, Anaplasma phagocytophilum, and Babesia spp. in patients with Tick-borne encephalitis. European J Clin Microbiol Infect Dis. 2014; 33: 1835–1841.
12.
Zhang XC, Yang ZN, Lu B, Ma XF, Zhang CX, Xu HJ. The composition and transmission of microbiome in hard tick, Ixodes persulcatus, during blood meal. Ticks Tick Borne Dis. 2014; 5: 864–870.
13.
Qiu Y, Nakao R, Ohnuma A, Kawamori F, Sugimoto C. Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLOS One. 2014; 9(8): e103961.
14.
Gerhart JG, Dutcher HA, Brenner AE, Moses AS, Grubhoffer L, Raghavan R. Multiple acquisitions of pathogen-derived Francisella endosymbionts in soft ticks. Genome Biol Evol. 2018; 10(2): 607–615.
15.
Duron O, Noel V, McCoy KD, Bonazzi M, Sidi-Boumedine K, Morel O, et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015; 11:e1004892.
16.
Narasimhan S, Fikrig E. Tick microbiome: the force within. Trends in Parasitology.2015; 31(7):315–323.
17.
Swei A, Kwan JY. Tick microbiome and pathogen acquisition altered by host blood meal. ISME J. 2017; 11: 813–816.
18.
Gall CA, Scoles GA, Magori K, Mason KL, Brayton KA. Laboratory colonization stabilizes the naturally dynamic microbiome composition of field collected Dermacentor andersoni ticks. Microbiome. 2017; 5: 133.
19.
Van Treuren W, Ponnusamy L, Brinkerhoff RJ, Gonzalez A, Parobek CM, Juliano JJ, et al. Variation in the microbiota of Ixodes ticks with regard to geography, species, and sex. Appl Environ Microbiol. 2015; 81(18): 6200–6209.
20.
Gofton AW, Oskam CL, Lo N, Beninati T, Wei H, McCari V, et al. Inhibition of the endosymbiont “Candidatus Midchloria mitochondrii” 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasite Vectors. 2015; 8:345.
21.
Panetta JL, Šima R, Calvani NE, Hajdušek O, Chandra S, Panuccio J, Šlapeta J. Reptile-associated Borrelia species in the goanna tick (Bothriocroton undatum) from Sydney, Australia. Parasite Vectors. 2017; 10:616.
22.
Kindworth A, PruesseE, Schweer T, Peplies J, Quast C, Horn M, Glöcker O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation-sequencing- based diversity studies. Nucleic Acid Res. 2013; 41(1): p11.
23.
Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, et al. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc Natl Acad Sci U.S.A. 2017; 114: E781–E790.
24.
Földvári G, Široký P, Szekeres S, Majoros G, Sprong H. Dermacentor reticulatus: a vector on the rise. Parasit Vectors. 2016; 9:314: 1–29.
25.
Rudolf I, Mendel J, Sikutová S, Svec P, Masaňiková J, Nováková D. 16SrRNA gene-based identification of cultured bacterial flora from host-seeking Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinn ticks, vectors of vertebrate pathogens. Folia Microbiologica (Praha). 2009; 54: 419–428.
26.
Sui S, Yang Y, Sun Y, Wang X, Wang G, Shan G, et al. On the core bacterial flora of Ixodes persulcatus (Taiga tick). PLOS One. 2017; 12(7): e0180150.
27.
Thapa S, Zhang Y, Allen MS. Effects of temperature on bacterial microbiome composition in Ixodes scapularis ticks. Microbiology Open. 2018; e719.
28.
Michalik J, Wodecka B, Liberska J, Dabert M, Postawa T, Piksa K, Stańczak J. Diversity of Borrelia burgdorferi sensu lato species in Ixodes ticks (Acari: Ixodidae) associated with cave-dwelling bats from Poland and Romania. Ticks Tick Borne Dis. 2020; 11(1):101300.
29.
Rudenko N, Golovchenko M, Lin T, Gao L, Grubhoffer L, Olivej J H Jr. Delineation of a new species of Borrelia burgdorferi sensu lato complex Borrelia americana sp. nov. J Clin Microbiol. 2009; 47:3875–3880.
30.
Mierzejewska EJ, Pawełczyk A, Radkowski M, Welc-Falęciak R, Bajer A. Pathogens vectored by the tick Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasit Vectors. 2015; 8: 490, 1–16.
31.
Wójcik-Fatla A, Cisak E, Zając V, Sroka J, Sawczyn A, Dutkiewicz J. Study on tick-borne rickettsiae in eastern Poland and prevalence in Dermacentor reticulatus (Acari: Amblyommidae). Ann Agric Environ Med. 2013; 20: 276–279.
32.
Stańczak J. Detection of spotted fever group (SFG) rickettsiae in Dermacentor reticulatus (Acari: Ixodidae) in Poland. Int J Med Microbiol. 2006; 296 Suppl. 40: 144–148.
33.
Chmielewski T, Podsiadły E, Karbowiak G, Tylewska-Wierzbanowska S. Rickettsia spp. in ticks, Poland. Emerg Infect Dis. 2009; 15: 486–488.
34.
Tijsse-Klasen E, Hansford KM, Jahfari S, Phipps P, Sprong H, Medlock JM. Spotted fever group rickettsiae in Dermacentor reticulatus and Haemaphysalis punctate ticks in the UK. Parasit Vectors. 2013;19(6): 212.
35.
Ponnusamy L, Gonzalez A, Van Treuren W, Weiss S, Parobek CM, Juliano JJ, et al. Diversity of Rickettsiales in the microbiome of the lone star tick, Amblyomma americanum. Appl Environ Microbiol. 2013; 80(1): 354–359.
36.
Greay TL, Gofton AW, Paparini A, Ryan UM, Oskam CL, Irwin PJ. Recent insights into the tick microbiome gained through next-generation sequencing. Parasit Vectors. 2018;11(1):12.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.