Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Extracellular matrix metalloproteinases in pathophysiology, diagnostics and treatment
of renal cell carcinoma – current state of knowledge and future perspectives
1
Department of Urology and Urological Oncology with Center for Treatment of Urolithiasis, Stefan Wyszynski Regional Specialist Hospital, Lublin, Poland
2
Department of Veterinary Hygiene, Provincial Veterinary Inspectorate, Lublin, Poland
3
Department of Neonatology, Pathology and Neonatal Intensive Care, Stefan Wyszynski Regional Specialist Hospital, Lublin, Poland
4
Department of Neonatology and Neonatal Intensive Care, Independent Public Specialist Hospital, Puławy, Poland
Corresponding author
Sławomir Jan Wątroba
Department of Neonatology and Neonatal Intensive Care, Independent Public Specialist Hospital, Puławy, Bema 1, 24-100, Puławy, Poland
Department of Neonatology and Neonatal Intensive Care, Independent Public Specialist Hospital, Puławy, Bema 1, 24-100, Puławy, Poland
Ann Agric Environ Med. 2025;32(1):27-45
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Despite a significant improvement in the diagnosis and treatment of renal cell carcinoma over the past two decades, this cancer still remains one of the most lethal urological neoplasms.
Review methods:
Databases were searched using the keywords: ‘RCC’, ‘MMP’, ‘TIMP’, ‘signaling pathway’, and ‘pathophysiological’. The titles of these entries were analyzed to assess complementarity, obtaining 721 entries. Relevance was then assessed by analyzing the abstracts, and 387 entries were selected. Based on criteria such as number of citations, research methods, sample size and representativeness, 248 references were finally selected. Finally, 181 items were included in the literature.
Brief description of the state of knowledge:
Extracellular matrix metalloproteinases plays an important role in the remodeling of the extracellular matrix. They participate in the initiation and regulation of inflammatory and carcinogenic processes.
Summary:
The analysis of available literature shows that, from a biochemical point of view, the most important influence on the development of renal cell carcinoma and the formation of metastases is the imbalance between the activity of metalloproteinases and the concentration of their tissue inhibitors. Hope for the future lies in monoclonal antibodies used to selectively block individual metalloproteinases, which may be of particula importance in highly vascularized tumours, which undoubtedly include renal cell carcinoma. Additionally, assessment of metalloproteinases activity could assist in selecting patients for surgical treatment or active surveillance.
Despite a significant improvement in the diagnosis and treatment of renal cell carcinoma over the past two decades, this cancer still remains one of the most lethal urological neoplasms.
Review methods:
Databases were searched using the keywords: ‘RCC’, ‘MMP’, ‘TIMP’, ‘signaling pathway’, and ‘pathophysiological’. The titles of these entries were analyzed to assess complementarity, obtaining 721 entries. Relevance was then assessed by analyzing the abstracts, and 387 entries were selected. Based on criteria such as number of citations, research methods, sample size and representativeness, 248 references were finally selected. Finally, 181 items were included in the literature.
Brief description of the state of knowledge:
Extracellular matrix metalloproteinases plays an important role in the remodeling of the extracellular matrix. They participate in the initiation and regulation of inflammatory and carcinogenic processes.
Summary:
The analysis of available literature shows that, from a biochemical point of view, the most important influence on the development of renal cell carcinoma and the formation of metastases is the imbalance between the activity of metalloproteinases and the concentration of their tissue inhibitors. Hope for the future lies in monoclonal antibodies used to selectively block individual metalloproteinases, which may be of particula importance in highly vascularized tumours, which undoubtedly include renal cell carcinoma. Additionally, assessment of metalloproteinases activity could assist in selecting patients for surgical treatment or active surveillance.
ABBREVIATIONS
APRF – factor of acute phase response; bFGF2 – basic fibroblast growth factor 2; BMI – body mass index; BMP-2 – bone morphogenetic protein 2; β-ct – catenin β; CAF – cancer-associated fibroblasts; ccRCC – clear cell renal cell carcinoma; CD63-R – CD63 receptor; CG – cathepsin G; chRCC – chromophobe renal cell carcinoma; CLDN-1 – claudin-1; COX-2 – cyclooxygenase 2; CT-α – chymotrypsin α; DBP – diastolic blood pressure; ECM – extracellular matrix; EMMPRIN – extracellular inducer of MMPs; EMT – mesenchymal-epithelial transition; EPIC – European
Prospective Investigation into Cancer and Nutrition; FAK – focal adhesion kinase; Fas-L – ligand Fas; FGF – fibroblast growth factor; GA – glutamic acid; GCs – glucocortikoids; GCTB – giant cell tumour of bone; HGF – hepatocyte growth factor; HIF – hypoxia-inducible factor; HO-1 – heme oxygenase-1; ICI – checkpoint inhibitor; IFN-β – interferon β; IFN-γ – interferon γ; IL-10 – interleukin 10; IL-12 – interleukin 12; Il-1β – interleukin 1β; IL-8/CXCL-8 – interleukin 8; IκBα – nuclear factor kappa-light-chain-enhancer of activated B cells type α; LPS – lipopolysaccharide; MAPK – mitogen-activated protein kinase; MMP – matrix metalloptoteinase; MNRN2 – multimerin 2; NF-κB – nuclear factor kappa-light-chain-enhancer of activated B cells; NQO1 – quinone oxidoreductase-1; NRF-2-ARE – nuclear factor erythroid-2-related factor 2/antioxidant response element; NSCLC – non-small cell lung cancer; PAR – poly-ADP-ribose; PDGF – platelet-derived growth factor; PI-3-K – phosphoinositide 3-kinase; PIAS – protein-activated STAT inhibitor; PLC – phospholipase C; pRCC – papillary renal cell carcinoma; p120-ct – catenin p120; RCC – renal cell carcinoma; SBP – systolic blood pressure; SOCS – suppressor cytokine signalling proteins; sRCC – sarcomatoid renal cell carcinoma; SRM – small renal tumour; TAM – cancer-associated macrophag; TC-PTP – T-cell protein tyrosine phosphatase TC45; TGF-β – transforming growth factor β; TIMP – tissue inhibitor of matrix metalloproteinases; TKIs – tyrosine kinase inhibitors; TNF – tumor necrosis factor; TR – trypsin; Tr-3-M/Tp-5-M – γ-3-monooxygenase tyrosine/5-monooxygenase tryptophan activating protein; uPAR – urokinase-type plasminogen activator receptor; VEGF – vascular endothelial growth factor; VEGFR – receptor of vascular endothelial growth factor; VHL – von Hippel-Lindau protein
REFERENCES (181)
1.
Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. https://doi.org/10.1016/j.euru....
2.
Pajunen H, Veitonmäki T, Huhtala H, et al. Prognostic factors of renal cell cancer in elderly patients: a population-based cohort study. Sci Rep. 2024;6295(14). https://doi.org/10.1038/s41598....
3.
Vamesu S, Ursica OA, Milea SE, et al. Same organ, two cancers: complete analysis of renal cell carcinomas and upper tract urothelial carcinomas. Medicina. 2024;60(7):1126. https://doi.org/10.3390/medici....
4.
Decastro GJ, McKiernan JM. Epidemiology, clinical staging, and presentation of renal cell carcinoma. Urol Clin North Am. 2008;35(4):581–592. https://doi.org/10.1016/j.ucl.....
5.
Falcão G, Parmanande AQ, Araújo C, et al. Bellini duct carcinoma. Autops Case Rep. 2020;11:e2020230. https://doi.org/10.4322/acr.20....
6.
Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal,penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. https://doi.org/10.1016/j.euru....
7.
Novacescu D, Feciche BO, Cumpanas AA, et al. Contemporary clinical definitions, differential diagnosis, and novel predictive tools for renal cell carcinoma. Biomedicines. 2022;10(11):2926. https://doi.org/10.3390/biomed....
8.
Rangasamy L, Geronimo BD, Ortín I, et al. Molecular imaging probes based on matrix metalloproteinase inhibitors (MMPIs). Molecules. 2019;24(16):2982. https://doi.org/10.3390/molecu....
9.
Xu Y, Yang Q, Fang Z, Tan X, et al. TRIM66 promotes malignant progression of non-small-cell lung cancer cells via targeting MMP9. Comput Math Methods Med. 2022;2022:6058720. https://doi.org/10.1155/2022/6....
10.
He Y, Zhou Y, Zhang J, et al. Tumor immunohistochemistry and preoperative magnetic resonance imaging features predict local recurrence of giant cell tumor of bone following intralesional curettage. Oncol Lett. 2019;17(2):1425–1434. https://doi.org/10.3892/ol.201....
11.
Li W, Ding Z, Wang D, et al. Ten-gene signature reveals the significance of clinical prognosis and immuno-correlation of osteosarcoma and study on novel skeleton inhibitors regarding MMP9. Cancer Cell Int. 2021;21(1):377. https://doi.org/10.1186/s12935....
12.
Jeleniewicz W, Cybulski M, Nowakowski A, et al. MMP-2 mRNA expression in ovarian cancer tissues predicts patients’ response to platinum-taxane chemotherapy. Anticancer Res. 2019;39(4):1821–1827. https://doi.org/10.21873/antic....
13.
Cheng Y, Cheng T, Zhao Y, et al. HMGA1 exacerbates tumor progression by activating miR-222 through PI3K/Akt/MMP-9 signaling pathway in uveal melanoma. Cell Signal. 2019;63:109386. https://doi.org/10.1016/j.cell....
14.
Huang H. Matrix Metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors (Basel). 2018;18(10):3249. https://doi.org/10.3390/s18103....
15.
Wu WC, Lee WJ, Lee TH, et al. Do different bariatric surgical procedures influence plasma levels of matrix metalloproteinase-2, -7, and -9 among patients with type 2 diabetes mellitus? World J Diabetes. 2020;11(6):252–260. https://doi.org/10.4239/wjd.v1....
16.
Kozakova M, Morizzo C, Goncalves I, et al. Cardiovascular organ damage in type 2 diabetes mellitus: the role of lipids and inflammation. Cardiovasc Diabetol. 2019;18(1):61. https://doi.org/10.1186/s12933....
17.
Goodwin LA. Closer look at metalloproteinases. Hauppauge, New York: Nova Science Publisher; 2019. p. 310.
18.
de Almeida LGN, Thode H, Eslambolchi Y, et al. Matrix metalloproteinases: from molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharmacol Rev. 2022;74(3):712–768. https://doi.org/10.1124/pharmr....
19.
Baidya SK, Banerjee S, Adhikari N, et al. Selective inhibitors of medium-size S1’ pocket matrix metalloproteinases: A stepping stone of future drug discovery. J Med Chem. 2022;65(16):10709–10754. https://doi.org/10.1021/acs.jm....
20.
Lin H, Xu P, Huang M. Structure-based molecular insights into matrix metalloproteinase inhibitors in cancer treatments. Future Med Chem. 2022;14(1):35–51. https://doi.org/10.4155/fmc-20....
21.
Jin Y, Eum DY, Lee C, et al. Breast cancer malignancy is governed by regulation of the macroH2A2/TM4SF1 axis, the AKT/NF-κB pathway, and elevated MMP13 expression. Mol Carcinog. 2024;63(4):714–727. https://doi.org/10.1002/mc.236.... https://doi.org/10.3390/cancer....
22.
Bayly-Jones C, Lupton CJ, Fritz C, et al. Helical ultrastructure of the metalloprotease meprin α in complex with a small molecule inhibitor. Nat Commun. 2022;13(1):6178. https://doi.org/10.1038/s41467....
23.
Wu S, Zhou X, Jin, Cheng H. Collagenases and their inhibitors: a review. Collagen & Leather. 2023;5:19. https://doi.org/10.1186/s42825....
24.
Afoshin A, Tishchenko S, Gabdulkhakov A, et al. Structural and functional characterization of β-lytic protease from lysobacter capsici VKM B-2533T. Int J Mol Sci. 2022;23(24):16100. https://doi.org/10.3390/ijms23....
25.
Varghese A, Chaturvedi SS, DiCastri B, et al. Effects of the nature of the metal ion, protein and substrate on the catalytic center in matrix metalloproteinase-1: Insights from multilevel MD, QM/MM and QM studies. Chemphyschem. 2021;23(4):10.1002/cphc.202100680. https://doi.org/10.1002/cphc.2....
26.
Gimeno A, Beltrán-Debón R, Mulero M, et al. Understanding the variability of the S1’ pocket to improve matrix metalloproteinase inhibitor selectivity profiles. Drug Discov Today. 2020;25(1):38–57. https://doi.org/10.1016/j.drud....
27.
Camacho E, Escalante T, Remans K, et al. Site mutation of residues in a loop surrounding the active site of a PI snake venom metalloproteinase abrogates its hemorrhagic activity. Biochem Biophys Res Commun. 2019;512:859–863.
28.
Varghese A, Chaturvedi SS, Fields GB, et al. A synergy between the catalytic and structural Zn(II) ions and the enzyme and substrate dynamics underlies the structure-function relationships of matrix metalloproteinase collagenolysis. J Biol Inorg Chem. 2021;26(5):583–597. https://doi.org/10.1007/s00775....
29.
Dolmatov IY, Nizhnichenko VA, Dolmatova LS. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in echinoderms: structure and possible functions. Cells. 2021;10(9):2331. https://doi.org/10.3390/cells1....
30.
Kim IS, Yang WS, Kim CH. Physiological properties, functions, and trends in the matrix metalloproteinase inhibitors in inflammation-mediated human diseases. Curr Med Chem. 2023;30(18):2075–2112. https://doi.org/10.2174/092986....
31.
Anuar NNM, Zulkafali NIN, Ugusman A. Modulation of matrix metalloproteinases by plant-derived products. Curr Cancer Drug Targets. 2021;21(2):91–106. https://doi.org/10.2174/156800....
32.
Madzharova E, Kastl P, Sabino F, et al. Post-translational modification-dependent activity of matrix metalloproteinases. Int J Mol Sci. 2019;20(12):3077. https://doi.org/10.3390/ijms20....
33.
Wątroba S, Wiśniowski T, Bryda J, et al. The role of matrix metalloproteinases in pathogenesis of human bladder cancer. Acta Biochim Pol. 2021;68(4):547–555. https://doi.org/10.18388/abp.2....
34.
Wątroba S, Wiśniowski T, Bryda J, et al. Characteristics of matrix metalloproteinases and their role in embryogenesis of the mammalian respiratory system. Postepy Hig Med Dosw. 2021;75:24–34. https://doi.org/10.5604/01.300....
35.
Wiśniowski T, Bryda J, Wątroba S. The role of matrix metalloproteinases in pathogenesis, diagnostics, and treatment of human prostate cancer. Postepy Hig Med Dosw. 2023;77:9–20. https://doi.org/10.2478/ahem-2....
36.
Wątroba S, Kocot J, Bryda J, et al. Serum activity of MMP-2 and MMP-9 and stromielisin-1 concentration as predictors in the pathogenesis of bronchopulmonary dysplasia in preterm neonates. Postepy Hig Med Dosw. 2019;73:703–712. https://doi.org/10.5604/01.300....
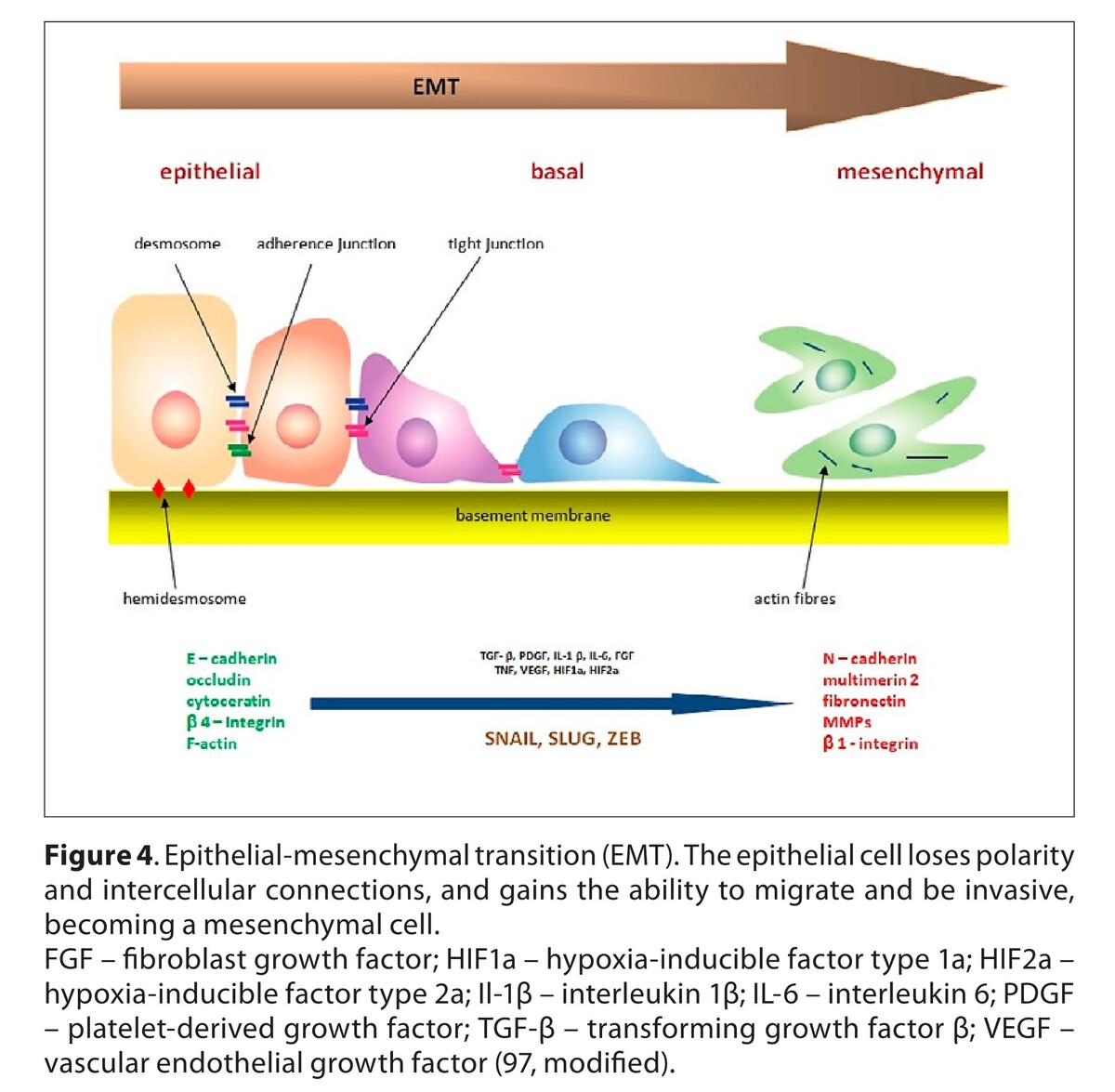
37.
Niland S, Riscanevo AX, Andreas J. Matrix metalloproteinases shape the tumor microenvironment in ancer progression. Int J Mol Sci. 2022;23(1):146. https://doi.org/10.3390/ijms23....
38.
Shan L, Wang F, Zhai D, et al. Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis. Biomed Pharmacother. 2023;161:114472. https://doi.org/10.1016/j.biop....
39.
Logue T, Lizotte-Waniewski M, Brew K. Thermodynamic profiles of the interactions of suramin, chondroitin sulfate, and pentosan polysulfate with the inhibitory domain of TIMP-3. FEBS Lett. 2020;594(1):94–103. https://doi.org/10.1002/1873-3....
40.
Escalona RM, Kannourakis G, Findlay JK, et al. Expression of TIMPs and MMPs in ovarian tumors, ascites, ascites-derived cells, and cancer cell lines: characteristic modulatory response before and after chemotherapy treatment. Front Oncol. 2022;11:796588. https://doi.org/10.3389/fonc.2....
41.
Costa S, Ragusa MA, Lo Buglio G, et al. The repertoire of tissue inhibitors of metalloproteases: evolution, regulation of extracellular matrix proteolysis, engineering and therapeutic challenges. Life (Basel). 2022;12(8):1145. https://doi.org/10.3390/life12....
42.
Charzewski Ł, Krzyśko KA, Lesyng B. Structural characterisation of inhibitory and non-inhibitory MMP-9-TIMP-1 complexes and implications for regulatory mechanisms of MMP-9. Sci Rep. 2021;11(1):13376. https://doi.org/10.1038/s41598....
43.
Malemud CJ. Inhibition of MMPs and ADAM/ADAMTS. Biochem Pharmacol. 2019; 165: 33–40. https://doi.org/10.1016/j.bcp.....
44.
Favaloro EJ, Henry BM, Lippi G. Increased VWF and decreased ADAMTS-13 in COVID-19: creating a milieu for (Micro) thrombosis. Semin Thromb Hemost. 2021;47(4):400–418. https://doi.org/10.1055/s-0041....
45.
Xia HQ, Yang JR, Zhang KX, et al. Molecules related to diabetic retinopathy in the vitreous and involved pathways. Int J Ophthalmol. 2022;15(7):1180–1189. https://doi.org/10.18240/ijo.2....
46.
Cambier S, Gouwy M, Proost P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towardspharmacological intervention. Cell Mol Immunol. 2023;20(3):217–251. https://doi.org/10.1038/s41423....
47.
Zhang Z, Wang H, Jin Y, et al. Potential of blood exosomal ENAH, SEPT9, EGF, MMP-9 and CXCL8 for the early screening of breast cancer. Oncol Lett. 2022;24(6):460. https://doi.org/10.3892/ol.202....
48.
Cardoso LM, Pansani TN, de Souza Costa CA, et al. Regulation of interleukin-6 and matrix metalloproteinases syntheses by bioflavonoids and photobiomodulation in human gingival fibroblasts. Lasers Med Sci. 2022;37(7):2973–2987. https://doi.org/10.1007/s10103....
49.
Gao H, Li Y, Chen X. Interactions between nuclear receptors glucocorticoid receptor α and peroxisome proliferator-activated receptor α form a negative feedback loop. Rev Endocr Metab Disord. 2022;23(5):893–903. https://doi.org/10.1007/s11154....
50.
Harsono AD, Prasetyono TOH, Dilogo IH. The Role of interleukin 10 in keloid therapy: a literature review. Ann Plast Surg. 2022;88(6):617–621. https://doi.org/10.1097/SAP.00....
51.
Lai JY, Ho JX, Kow ASF, et al. Interferon therapy and its association with depressive disorders – A review. Front Immunol. 2023;14:1048592. https://doi.org/10.3389/fimmu.....
52.
Li S, Pritchard DM, Yu LG. Regulation and function of matrix metalloproteinase-13 in cancer progression and metastasis. Cancers (Basel). 2022;14(13):3263. https://doi.org/10.3390/cancer....
53.
Wan J, Zhang G, Li X, et al. Matrix metalloproteinase 3: a promoting and destabilizing factor in the pathogenesis of disease and cell differentiation. Front Physiol. 2021;12:663978. https://doi.org/10.3389/fphys.....
54.
Yu J, Hu G, Cao H, et al. Quercetin ameliorates lipopolysaccharide-induced duodenal inflammation through modulating autophagy, programmed cell death and intestinal mucosal barrier function in chicken embryos. Animals (Basel). 2022;12(24):3524. https://doi.org/10.3390/ani122....
55.
Hannocks MJ, Zhang X, Gerwien H, et al. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019;75–76:102–113. https://doi.org/10.1016/j.matb....
56.
Zhou W, Yu X, Sun S, et al. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed Pharmacother. 2019;118:109369. https://doi.org/10.1016/j.biop....
57.
Henriet P, Emonard H. Matrix metalloproteinase-2: Not (just) a “hero” of the past. Biochimie. 2019;166:223–232. https://doi.org/10.1016/j.bioc....
58.
Hey S, Ratt A, Linder S. There and back again: Intracellular trafficking, release and recycling of matrix metalloproteinases. Biochim Biophys Acta Mol Cell Res. 2022;1869(4):119189. https://doi.org/10.1016/j.bbam....
59.
Grillet B, Pereira RVS, Van Damme J, et al. Matrix metalloproteinases in arthritis: towards precision medicine. Nat Rev Rheumatol. 2023;19(6):363–377. https://doi.org/10.1038/s41584....
60.
García-López C, Rodríguez-Calvo-de-Mora M, Borroni D, et al. The role of matrix metalloproteinases in infectious corneal ulcers. Surv Ophthalmol. 2023;68(5):929–939. https://doi.org/10.1016/j.surv....
61.
Hamdi T. What is the function of matrix metalloproteinase-2 and matrix metalloproteinase-9 in pain processes? JSOCMED. 2023;2:49–53.
62.
Rodriguez-Rios M, Megia-Fernandez A, Norman DJ, et al. Peptide probes for proteases – innovations and applications for monitoring proteolytic activity. Chem Soc Rev. 2022;51(6):2081–2120. https://doi.org/10.1039/d1cs00....
63.
Minor AJ, Coulombe KLK. Engineering a collagen matrix for cell-instructive regenerative angiogenesis. J Biomed Mater Res B Appl Biomater. 2020;108(6):2407–2416. https://doi.org/10.1002/jbm.b.....
64.
Tavianatou AG, Caon I, Franchi M, et al. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019;286(15):2883–2908. https://doi.org/10.1111/febs.1....
65.
Laronha H, Caldeira J. Structure and function of human matrix metalloproteinases. Cells. 2020;9(5):1076. https://doi.org/10.3390/cells9....
66.
Jamerson EC, Elhusseiny AM, ElSheikh RH, et al. Role of matrix metalloproteinase 9 in ocular surface isorders. Eye Contact Lens. 2020;46:S57–S63. https://doi.org/10.1097/ICL.00....
67.
Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, et al. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front Immunol. 2019;10:238. https://doi.org/10.3389/fimmu.....
68.
Alamgeer ST, Hasan UH, Uttra AM, et al. Phytochemicals targeting matrix metalloproteinases regulating tissue degradation in inflammation and rheumatoid arthritis. Phytomedicine. 2020;66:153134. https://doi.org/10.1016/j.phym....
69.
Nanda A, Thangapandi K, Banerjee P, et al. Cytokines, angiogenesis, and extracellular matrix degradation are augmented by oxidative stress in endometriosis. Ann Lab Med. 2020;40(5):390–397. https://doi.org/10.3343/alm.20....
70.
Weyand CM, Watanabe R, Zhang H, et al. Cytokines, growth factors and proteases in medium and large vessel vasculitis. Clin Immunol. 2019;206:33–41. https://doi.org/10.1016/j.clim....
71.
Luchian I, Goriuc A, Sandu D, et al. The role of matrix metalloproteinases (MMP-8, MMP-9, MMP-13) in periodontal and peri-implant pathological processes. Int J Mol Sci. 2022;23(3):1806. https://doi.org/10.3390/ijms23....
72.
Maybee DV, Ink NL, Ali MAM. Novel roles of MT1-MMP and MMP-2: beyond the extracellular milieu. Int J Mol Sci. 2022;23(17):9513. https://doi.org/10.3390/ijms23....
73.
Haas NB, Song Y, Willemann-Rogerio J, et al. Disease-free survival as a predictor of overall survival in localized renal cell carcinoma following initial nephrectomy: A retrospective analysis of surveillance, epidemiology and end results-medicare datac. Int J Urol. 2023;30(3):272–279. https://doi.org/10.1111/iju.15....
74.
Murray AA, Gallegos JAO, Jones S, et al. Epidemiological characterization of renal cell carcinoma in Hispanics: A single US center cohort study. Cancer Res. 2023;83:722. https://doi.org/10.1158/1538-7....
75.
Posada Calderon L, Eismann L, Reese SW, et al. Advances in imaging-based biomarkers in renal cell carcinoma: a critical analysis of the current literature. Cancers (Basel). 2023;15(2):354. https://doi.org/10.3390/cancer....
76.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.2....
77.
Orczykowski M, Tyszkiewicz M, Rosińska A, et al. Renal cell carcinoma – epidemiology, risk factors, diagnosis and treatment – review article. J Edu Health Sport. 2022;13:275–280. https://doi.org/10.12775/JEHS.....
78.
Al-Bayati O, Hasan A, Pruthi D, et al. Systematic review of modifiable risk factors for kidney cancer. Urol Oncol. 2019;37(6):359–371. https://doi.org/10.1016/j.urol....
79.
Tsivian M, Moreira DM, Caso JR, et al. Cigarette smoking is associated with advanced renal cell carcinoma. J Clin Oncol. 2011;29(15):2027–2031. https://doi.org/10.1200/JCO.20....
80.
Callahan CL, Hofmann JN, Corley DA, et al. Obesity and renal cell carcinoma risk by histologic subtype: A nested case-control study and meta-analysis. Cancer Epidemiol. 2018;56:31–37. https://doi.org/10.1016/j.cane....
81.
Tahbaz R, Schmid M, Merseburger AS. Prevention of kidney cancer incidence and recurrence: lifestyle, medication and nutrition. Curr Opin Urol. 2018;28(1):62–79. https://doi.org/10.1097/MOU.00....
82.
Humphrey PA, Moch H, Cubilla AL, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–119. https://doi.org/10.1016/j.euru....
83.
Chevrier S, Levine JH, Zanotelli VRT, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;69(4):736–749. https://doi.org/10.1016/j.cell....
84.
Klatte T, Rossi SH, Stewart GD. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J Urol. 2018;36(12):1943–1952. https://doi.org/10.1007/s00345....
85.
Mazumder S, Higgins PJ, Samarakoon R. Downstream targets of VHL/HIF-α signaling in renal clear cell carcinoma progression: mechanisms and therapeutic relevance. Cancers (Basel). 2023;15(4):1316. https://doi.org/10.3390/cancer....
86.
Yokoji-Takeuchi M, Takahashi N, Yamada-Hara M, et al. A bacterial metabolite induces Nrf2-mediated anti-oxidative responses in gingival epithelial cells by activating the MAPK signaling pathway. Arch Oral Biol. 2020;110:104602. https://doi.org/10.1016/j.arch....
87.
Lu CW, Lee CJ, Hsieh YJ, et al. Empagliflozin attenuates vascular calcification in mice with chronic kidney disease by regulating the NFR2/HO-1 anti-inflammatory pathway through AMPK activation. Int J Mol Sci. 2023;24(12):10016. https://doi.org/10.3390/ijms24....
88.
Sulijaya B, Takahashi N, Yamada M, et al. The anti-inflammatory effect of 10-oxo-trans-11-octadecenoic acid (KetoC) on RAW 264.7 cells stimulated with Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. 2018;53(5):777–784. https://doi.org/10.1111/jre.12....
89.
Linehan WM, Spellman PT, Ricketts CJ, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135–145. https://doi.org/10.1056/NEJMoa....
90.
Prasad SR, Humphrey PA, Catena JR, et al. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006;26(6):1795–806. https://doi.org/10.1148/rg.266....
91.
Ohashi R, Schraml P, Angori S, et al. Classic chromophobe renal cell carcinoma incur a larger number of chromosomal losses than seen in the eosinophilic subtype. Cancers (Basel). 2019;11(10):1492. https://doi.org/10.3390/cancer....
92.
Delahunt B, Eble JN, Egevad L, et al. Grading of renal cell carcinoma. Histopathology. 2019;74(1):4–17. https://doi.org/10.1111/his.13....
93.
Swami U, Nussenzveig RH, Haaland B, et al. Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement. Ann Transl Med. 2019;7:S18. https://doi.org/10.21037/atm.2....
94.
Capitanio U, Bedke J, Albiges L, et al. A renewal of the TNM staging system for patients with renal cancer to comply with current decision-making: proposal from the European Association of Urology Guidelines Panel. Eur Urol. 2023;83(1):3–5. https://doi.org/10.1016/j.euru....
95.
Shay G, Lynch CC, Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44–46:200–6. https://doi.org/10.1016/j.matb....
96.
Taddei ML, Giannoni E, Comito G, et al. Microenvironment and tumor cell plasticity: an easy way out. Cancer Lett. 2013;341:80–96. https://doi.org/10.1016/j.canl....
97.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. https://doi.org/10.1038/s41580....
98.
Khan T, Kryza T, Lyons NJ, et al. The CDCP1 signaling hub: a target for cancer detection and therapeutic intervention. Cancer Res. 2021;81(9):2259–2269. https://doi.org/10.1158/0008-5....
99.
Kessenbrock K, Wang CY, Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015;44–46:184–190. https://doi.org/10.1016/j.matb....
100.
Walker C, Mojares E, Del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19(10):3028. https://doi.org/10.3390/ijms19....
101.
Ostrowski PP, Freeman SA, Fairn G, et al. Dynamic podosome-like structures in nascent phagosomes are coordinated by phosphoinositides. Dev Cell. 2019;50(4):397–410. https://doi.org/10.1016/j.devc....
102.
Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12(7):413–426. https://doi.org/10.1038/nrm314....
103.
Seano G, Primo L. Podosomes and invadopodia: tools to breach vascular basement membrane. Cell Cycle. 2015;14(9):1370–1374. https://doi.org/10.1080/153841....
104.
Yan X, Cao N, Chen Y, et al. MT4-MMP promotes invadopodia formation and cell motility in FaDu head and neck cancer cells. Biochem Biophys Res Commun. 2020;522(4):1009–1014. https://doi.org/10.1016/j.bbrc....
105.
Gulvady AC, Forsythe IJ, Turner CE. Hic-5 regulates Src-induced invadopodia rosette formation and organization. Mol Biol Cell. 2019;30(11):1298–1313. https://doi.org/10.1091/mbc.E1....
106.
Petrella BL, Vincenti MP. Interleukin-1β mediates metalloproteinase-dependent renal cell carcinoma tumor cell invasion through the activation of CCAAT enhancer binding protein β. Cancer Med. 2012;1(1):17–27. https://doi.org/10.1002/cam4.7.
107.
Yamaguchi H, Sakai R. Direct interaction between carcinoma cells and cancer associated fibroblasts for the regulation of cancer invasion. Cancers (Basel). 2015;7(4):2054–2062. https://doi.org/10.3390/cancer....
108.
Erdogan B, Ao M, White LM, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216(11):3799–3816. https://doi.org/10.1083/jcb.20....
109.
Wang JP, Hielscher A. Fibronectin: How its aberrant expression in tumors may improve therapeutic targeting. J Cancer. 2017;8(4):674–682. https://doi.org/10.7150/jca.16....
110.
Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, et al. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57–83. https://doi.org/10.1016/j.crit....
111.
Choi JY, Jang YS, Min SY, et al. Overexpression of MMP-9 and HIF-1α in breast cancer cells under hypoxic conditions. J Breast Cancer. 2011;14(2):88–95. https://doi.org/10.4048/jbc.20....
112.
Kim H, Shim BY, Lee SJ, et al. Loss of von hippel-lindau (VHL) tumor suppressor gene function: VHL-HIF pathway and advances in treatments for metastatic renal cell carcinoma (RCC). Int J Mol Sci. 2021;22(18):9795. https://doi.org/10.3390/ijms22....
113.
Mengie Ayele T, Tilahun Muche Z, Behaile Teklemariam A, et al. Role of JAK2/STAT3 signaling pathway in the tumorigenesis, chemotherapy resistance, and treatment of solid tumors: a systemic review. J Inflamm Res. 2022;15:1349–1364. https://doi.org/10.2147/JIR.S3....
114.
Glassman CR, Tsutsumi N, Saxton RA, et al. Structure of a Janus kinase cytokine receptor complex reveals the basis for dimeric activation. Science. 2022;376(6589):163–169. https://doi.org/10.1126/scienc....
115.
Roskoski R Jr. Janus kinase (JAK) inhibitors in the treatment of neoplastic and inflammatory disorders. Pharmacol Res. 2022;183:106362. https://doi.org/10.1016/j.phrs....
116.
Porvahdani S, Baradaran B, Talebi M, et al. STATs inhibitors in cancer therapy strategies. Ann Hematol Oncol Res. 2022;2:1013.
117.
Aigner P, Just V, Stoiber D. STAT3 isoforms: Alternative fates in cancer? Cytokine. 2019;118:27–34. https://doi.org/10.1016/j.cyto....
118.
Pang Q, You L, Meng X, et al. Regulation of the JAK/STAT signaling pathway: The promising targets for cardiovascular disease. Biochem Pharmacol. 2023;213:115587. https://doi.org/10.1016/j.bcp.....
119.
Liu Y, Liao S, Bennett S, et al. STAT3 and its targeting inhibitors in osteosarcoma. Cell Prolif. 2021;54(2):e12974. https://doi.org/10.1111/cpr.12....
120.
Wang J, Gao T, Ma Y, et al. Discovery of unglycosylated indolocarbazoles as ROCK2 isoform-selective inhibitors for the treatment of breast cancer metastasis. Eur J Med Chem. 2023;250:115181. https://doi.org/10.1016/j.ejme....
121.
Ashrafizadeh M, Mohan CD, Rangappa S, et al. Noncoding RNAs as regulators of STAT3 pathway in gastrointestinal cancers: Roles in cancer progression and therapeutic response. Med Res Rev. 2023;43(5):1263–1321. https://doi.org/10.1002/med.21....
122.
Xiang C, Wu W, Fan M, et al. Phosphorylated STAT3 as a potential diagnostic and predictive biomarker in ALK- ALCL vs. CD30high PTCL, NOS. Front Immunol. 2023;14:1132834. https://doi.org/10.3389/fimmu.....
123.
Ashmawy AI, El-Abhar HS, Abdallah DM, et al. Corrigendum to “Chloroquine modulates the sulforaphane anti-obesity mechanisms in a high-fat diet model: Role of JAK-2/ STAT-3/ SOCS-3 pathway”. Eur J Pharmacol. 2023;947:175683. https://doi.org/10.1016/j.ejph....
124.
Rusek M, Smith J, El-Khatib K, et al. The role of the JAK/STAT signaling pathway in the pathogenesis of Alzheimer’s Disease: New potential treatment target. Int J Mol Sci. 2023;24(1):864. https://doi.org/10.3390/ijms24....
125.
Lang R, Raffi FAM. Dual-specificity phosphatases in immunity and infection: an update. Int J Mol Sci. 2019;20(11):2710. https://doi.org/10.3390/ijms20....
126.
Deng S, Wang C, Wang Y, et al. Ectopic JAK-STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat Cancer. 2022;3(9):1071–1087. https://doi.org/10.1038/s43018....
127.
Liu J, Wang F, Luo F. The role of JAK/STAT pathway in fibrotic diseases: molecular and cellular mechanisms. Biomolecules. 2023;13(1):119. https://doi.org/10.3390/biom13....
128.
Rah B, Rather RA, Bhat GR, et al. JAK/STAT signaling: molecular targets, therapeutic opportunities, and limitations of targeted inhibitions in solid malignancies. Front Pharmacol. 2022;13:821344. https://doi.org/10.3389/fphar.....
129.
Fahmideh H, Shapourian H, Moltafeti R, et al. The role of natural products as inhibitors of JAK/STAT signaling pathways in glioblastoma treatment. Oxid Med Cell Longev. 2022;2022:7838583. https://doi.org/10.1155/2022/7....
130.
García-Castellano JM, García-Padrón D, Martínez-Aragón N, et al. Role of the IL-6/Jak/Stat pathway in tumor angiogenesis: influence of estrogen status. 2022. IntechOpen. https://doi.org/10.5772/intech....
131.
Rajakumar T, Pugalendhi P. Allyl isothiocyanate inhibits invasion and angiogenesis in breast cancer via EGFR-mediated JAK-1/STAT-3 signaling pathway. Amino Acids. 2023;55(8):981–992. https://doi.org/10.1007/s00726....
132.
Roland CL, Lynn KD, Toombs JE, et al. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One. 2009;4(11):e7669. https://doi.org/10.1371/journa....
133.
Yu Y, Elble RC. Homeostatic signaling by cell-cell junctions and its dysregulation during cancer progression. J Clin Med. 2016;5(2):26. https://doi.org/10.3390/jcm502....
134.
Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796(2):75–90. https://doi.org/10.1016/j.bbca....
135.
Na TY, Schecterson L, Mendonsa AM, et al. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci U S A. 2020;117(11):5931–5937. https://doi.org/10.1073/pnas.1....
136.
Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):201–212. https://doi.org/10.1007/s10911....
137.
Shukla V, Adiga D, Jishnu PV, et al. Role of miRNA clusters in epithelial to mesenchymal transition in cancer. Front Biosci (Elite Ed). 2020;12(1):48–78. https://doi.org/10.2741/E857.
138.
Kazmi I, Alharbi KS, Al-Abbasi FA, et al. Role of epithelial-to-mesenchymal transition markers in different stages of endometriosis: expression of the snail, slug, ZEB1, and twist genes. Crit Rev Eukaryot Gene Expr. 2021;31(2):89–95. https://doi.org/10.1615/CritRe....
139.
Wu JE, Wu YY, Tung CH, et al. DNA methylation maintains the CLDN1-EPHB6-SLUG axis to enhance chemotherapeutic efficacy and inhibit lung cancer progression. Theranostics. 2020;10(19):8903–8923. https://doi.org/10.7150/thno.4....
140.
Zou X, Ma L, Zhang Y, et al. GATA zinc finger protein p66β promotes breast cancer cell migration by acting as a co-activator of Snail. Cell Death Dis. 2023;14(6):382. https://doi.org/10.1038/s41419.... Bi Y, Cui D, Xiong X, et al. The characteristics and roles of β-TrCP1/2 in carcinogenesis. FEBS J. 2021;288(11):3351–3374. https://doi.org/10.1111/febs.1....
141.
Bi Y, Cui D, Xiong X, et al. The characteristics and roles of β-TrCP1/2 in carcinogenesis. FEBS J. 2021;288(11):3351–337.
142.
Xu Y, Ye S, Zhang N, et al. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun (Lond). 2020;40(10):484–500. https://doi.org/10.1002/cac2.1....
143.
Yue CH, Chen CH, Pan YR, et al. Cetyltrimethylammonium bromide disrupts mesenchymal characteristics of human tongue squamous cell carcinoma SCC4 cells through modulating canonical TGF-β/Smad/miR-181b/TIMP3 signaling pathway. Anticancer Res. 2021;41(12):6095–6104. https://doi.org/10.21873/antic....
144.
Pliakou E, Lampropoulou DI, Dovrolis N, et al. Circulating miRNA expression profiles and machine learning models in association with response to irinotecan-based treatment in metastatic colorectal cancer. Int J Mol Sci. 2022;24(1):46. https://doi.org/10.3390/ijms24....
145.
Buyuk B, Jin S, Ye K. Epithelial-to-mesenchymal transition signaling pathways responsible for breast cancer metastasis. Cell Mol Bioeng. 2021;15(1):1–13. https://doi.org/10.1007/s12195....
146.
Gentilcore G, Madonna G, Mozzillo N, et al. Effect of dabrafenib on melanoma cell lines harbouring the BRAF(V600D/R) mutations. BMC Cancer. 2013;13:17. https://doi.org/10.1186/1471-2....
147.
Simpson CD, Anyiwe K, Schimmer AD. Anoikis resistance and tumor metastasis. Cancer Lett. 2008;272(2):177–185. https://doi.org/10.1016/j.canl....
148.
Frisch SM, Schaller M, Cieply B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J Cell Sci. 2013;126:21–29. https://doi.org/10.1242/jcs.12....
149.
Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta. 2013;1833(12):3481–3498. https://doi.org/10.1016/j.bbam....
150.
Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1(27):re6. https://doi.org/10.1126/scisig....
151.
Carey P, Low E, Harper E, et al. Metalloproteinases in ovarian cancer. Int J Mol Sci. 2021;22(7):3403. https://doi.org/10.3390/ijms22....
152.
Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745–1770. https://doi.org/10.1007/s00018....
153.
Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44–46:94–112. https://doi.org/10.1016/j.matb....
154.
Eiro N, González L, Martínez-Ordoñez A, et al. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell Oncol (Dordr). 2018;41(4):369–378. https://doi.org/10.1007/s13402....
155.
Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205. https://doi.org/10.1016/j.ccr.....
156.
Mazor R, Alsaigh T, Shaked H, et al. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Chem. 2013;288(1):598–607. https://doi.org/10.1074/jbc.M1....
157.
Gupta GP, Nguyen DX, Chiang AC, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446(7137):765–770. https://doi.org/10.1038/nature....
158.
Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739. https://doi.org/10.3390/ijms21....
159.
Zhao YW, Ma W, Jiang F, et al. Upregulation of matrix metalloproteinase 14 (MMP14) is associated with poor prognosis in renal clear cell carcinoma-a bioinformatics analysis. Transl Androl Urol. 2022;11(11):1523–1534. https://doi.org/10.21037/tau-2....
160.
Kushlinskii NE, Gershtein ES, Alferov AA, et al. Prognostic role of matrix metalloproteinases 2, 7, 8, 9 and their type 1 tissue inhibitor in blood serum of patients with kidney cancer. Bull Exp Biol Med. 2020;168(5):673–676. https://doi.org/10.1007/s10517....
161.
Gershtein ES, Mushtenko SV, Ermilova VD, et al. Matrix metalloproteinases and their tissue inhibitors in blood serum of patients with endometrial cancer: clinical and morphological correlations. Bull Exp Biol Med. 2018;165(1):75–79. https://doi.org/10.1007/s10517....
162.
Cho NH, Shim HS, Rha SY, et al. Increased expression of matrix metalloproteinase 9 correlates with poor prognostic variables in renal cell carcinoma. Eur Urol. 2003;44(5):560–566. https://doi.org/10.1016/s0302-....
163.
Bassiouni W, Ali MAM, Schulz R. Multifunctional intracellular matrix metalloproteinases: implications in disease. FEBS J. 2021;288(24):7162–7182. https://doi.org/10.1111/febs.1....
164.
Miyata Y, Iwata T, Maruta S, et al. Expression of matrix metalloproteinase-10 in renal cell carcinoma and its prognostic role. Eur Urol. 2007;52:791–797.
165.
Kageyama Y, Nakamura M, Igari Y, et al. Expression of matrix metalloproteinase-3 and -10 is up-regulated in the periodontal tissues of aged mice. J Periodontal Res. 2022;57(4):733–741. https://doi.org/10.1111/jre.12....
166.
Kudelski J, Młynarczyk G, Gudowska-Sawczuk M, et al. Higher content but not activity of stromelysin-2 (MMP-10) in comparison to stromelysin-1 (MMP-3) in human renal carcinoma. Int J Environ Res Public Health. 2022;19(19):12613. https://doi.org/10.3390/ijerph....
167.
Młynarczyk G, Kudelski J, Darewicz B, et al. Suppressed expression but not activity of collagenases MMP-1 and MMP-13 in human renal carcinoma. Pathobiology. 2019;86(4):201–207. https://doi.org/10.1159/000499....
168.
Moch H, Amin MB, Berney DM, et al. The 2022 world health organization classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2022;82950:458–468. https://doi.org/10.1016/j.euru....
169.
Chrabańska M, Rynkiewicz M, Kiczmer P, et al. Does the immunohistochemical expression of CD44, MMP-2, and MMP-9 in association with the histopathological subtype of renal cell carcinoma affect the survival of patients with renal cancer? Cancers (Basel). 2023;15(4):1202. https://doi.org/10.3390/cancer....
170.
Mehdi MZ, Nagi AH, Naseem N. MCM – 2 and Ki – 67 as proliferation markers in renal cell carcinoma: A quantitative and semi – quantitative analysis. Int Braz J Urol. 2016;42(6):1121–1128. https://doi.org/10.1590/S1677-....
171.
Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME randomized clinical trial. JAMA Oncol. 2019;5(2):164–170. https://doi.org/10.1001/jamaon....
172.
Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–427. https://doi.org/10.1056/NEJMoa....
173.
Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. https://doi.org/10.1056/NEJMoa....
174.
Fields GB. Mechanisms of action of novel drugs targeting angiogenesis-promoting matrix metalloproteinases. Front Immunol. 2019;10:1278. https://doi.org/10.3389/fimmu.....
175.
Laronha H, Carpinteiro I, Portugal J, et al. Challenges in matrix metalloproteinases inhibition. Biomolecules. 2020;10(5):717. https://doi.org/10.3390/biom10....
176.
Ling B, Watt K, Banerjee S, et al. A novel immunotherapy targeting MMP-14 limits hypoxia, immune suppression and metastasis in triple-negative breast cancer models. Oncotarget. 2017;8(35):58372–58385. https://doi.org/10.18632/oncot....
177.
Devy L, Huang L, Naa L, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69(4):1517–1526. https://doi.org/10.1158/0008-5....
178.
Ingvarsen S, Porse A, Erpicum C, et al. Targeting a single function of the multifunctional matrix metalloprotease MT1-MMP: impact on lymphangiogenesis. J Biol Chem. 2013;288(15):10195–10204. https://doi.org/10.1074/jbc.M1....
179.
Udi Y, Grossman M, Solomonov I, et al. Inhibition mechanism of membrane metalloprotease by an exosite-swiveling conformational antibody. Structure. 2015;23(1):104–115. https://doi.org/10.1016/j.str.....
180.
Yin J, Zhu W, Feng S, et al. The role of cancer-associated fibroblasts in the invasion and metastasis of colorectal cancer. Front Cell Dev Biol. 2024;30(12):1375543. https://doi: 10.3389/fcell.2024.1375543.
181.
Mattila KE, Vainio P, Jaakkola PM. Prognostic factors for localized clear cell renal cell carcinoma and their application in adjuvant therapy. Cancers (Basel). 2022;14(1):239. https://doi.org/10.3390/cancer....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.