Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
CASE REPORT
Disruptions within gut microbiota composition induced by improper antibiotics therapy as a probable trigger factor for development of depression – Case Reports
1
Ist Department of Psychiatry, Psychotherapy and Early Intervention, Medical University, Lublin, Poland
Corresponding author
Joanna Rog
Ist Department of Psychiatry, Psychotherapy and Early Intervention, Medical University of Lublin, Poland
Ist Department of Psychiatry, Psychotherapy and Early Intervention, Medical University of Lublin, Poland
Ann Agric Environ Med. 2021;28(4):713-718
KEYWORDS
gut microbiotamajor depressive disorderdepressionmood disorderprobioticsgut microbiomegut permeability
TOPICS
ABSTRACT
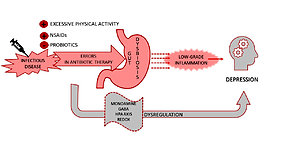
A growing body of evidence confirms that immune activation and low-grade inflammation could be defined as risks factors for the development and progression of mood episodes. A suggested mechanism leading to immune-inflammatory imbalance is the change in gut microbiota composition provoked by intestinal permeability. Three cases of patients with first episode of depression: a 30-year-old man and two 41 and 46-year-old women are presented in the study. In all cases, the episode was proceeded by the infection of the upper respiratory tract and improper antibiotics therapy. Despite the subsidence of infection, gastrointestinal and depression symptoms appeared. Psychiatric care, anti-depressant treatment, and probiotic supplementation were applied with positive results. Changes in gut microbiota and gut permeability are mechanisms probably involved in the development of mood disorders among the described patients. Lack of microbiota and gut permeability analysis allows defining just a temporary, potential cause-effect relationship between disease symptoms and intestinal microbiome alternations. Further studies to establish the importance of gut bacteria, immune-inflammatory cascade at depression etiopathogenesis and therapy are needed.
Janowska M, Rog J, Karakula-Juchnowicz H. Disruptions within gut microbiota composition induced by improper antibiotics therapy as a probable trigger factor for development of depression – Case Reports. Ann Agric Environ Med. doi: 10.26444/aaem/132452
REFERENCES (54)
1.
Dudek D, Siwek M. Współistnienie chorób somatycznych i depresji. Psychiatria. 2007; 4(1): 17–24.
2.
Moskalewicz J, Wciórka J, Stokwiszewski J, Rabczenko D, Kessler RC. The prevalence of common mental disorders in the population of adult Poles by sex and age structure–an EZOP Poland study. Psychiatr Pol. 2015; 49(1): 15–27.
3.
Jasik K, Jaślikowska U, Zbrojkiewicz M, et al. Czynniki związane z występowaniem depresji u osób dorosłych. Przegląd systematyczny literatury polskiej w latach 2009–2014 [Factors related to occurrence of depressive disorders in adults. A systematic review of Polish literature in years 2009–2014]. Journal of Education, Health and Sport. 2016; 6(4): 297–318.
4.
Wu H, Tremaroli V, Bäckhed F. Linking microbiota to human diseases: a systems biology perspective. Trends in Endocrinology & Metabolism. 2015; 26(12): 758–770.
5.
Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nature neuroscience. 2017; 20(2): 145.
6.
Lu J, Lu L, Yu Y, Cluette-Brown J, Martin CR, Claud EC. Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Scientific reports. 2018; 8(1): 1–16.
7.
Nakata K, Sugi Y, Narabayashi H, et al. Commensal microbiota-induced microRNA modulates intestinal epithelial permeability through the small GTPase ARF4. Journal of Biological Chemistry. 2017; 292(37): 15426–15433.
8.
Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science translational medicine. 2014; 6(263): 263ra158-263ra158.
9.
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Frontiers in cellular neuroscience. 2015; 9: 392.
10.
Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012; 19(2): 121–130.
11.
Kelly JR, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbiota axis: challenges for translation in psychiatry. Annals of Epidemiology. 2016; 26(5): 366 –372.
12.
Skonieczna-Żydecka K, Grochans E, Maciejewska D, et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients. 2018; 10(12): 1939.
13.
Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterology & Motility. 2014; 26(8): 1155–1162.
14.
Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain, behavior, and immunity. 2015; 48: 186–194.
15.
Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nature Medicine. 2017; 23(7): 850–858. doi: 10.1038/nm.4345.
16.
Liśkiewicz P, Pełka-Wysiecka J, Kaczmarczyk M, et al. Fecal Microbiota Analysis in Patients Going through a Depressive Episode during Treatment in a Psychiatric Hospital Setting. Journal of Clinical Medicine. 2019; 8(2): 164. doi:10.3390/jcm8020164.
17.
Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016; 8(8): 483.
18.
Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016; 32(3): 315–320.
19.
Köhler O, Petersen L, Mors O, Gasse C. Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain and behavior. 2015; 5(8): e00338.
20.
Ng QX, Peters C, Ho CYX, Lim DY, Yeo W-S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. Journal of affective disorders. 2018; 228: 13–19.
21.
Wojkowska-Mach J, Godman B, Glassman A, et al. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrobial Resistance & Infection Control. 2018; 7(1): 136. doi: 10.1186/s13756-018-0428-8.
22.
Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019; 5(2): eaau8317. doi: 10.1126/sciadv.aau8317.
23.
Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. 2018; 67(8): 1555–1557.
24.
Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterology & Motility. 2013; 25(9): 713–719.
25.
Karakula-Juchnowicz H, Gałęcka M, Rog J, et al. The food-specific serum IgG reactivity in major depressive disorder patients, irritable bowel syndrome patients and healthy controls. Nutrients. 2018; 10(5): 548.
26.
Karakuła-Juchnowicz H, Pankowicz H, Juchnowicz D. Psychobiotics: new possibilities for treatment of affective disorders? Pharmacotherapy in Psychiatry and Neurology. 2016; (2015 volume 31 issue 3–4): 229–242. doi: 10.17393/fpn.2016.01.005.
27.
Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychological medicine. 2019; 49(12): 1958–1970.
28.
Rogers MA, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clinical Microbiology and Infection. 2016; 22(2): 178-e1.
29.
Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophrenia Research. 2018; 201: 299–306. doi: 10.1016/j.schres.2018.05.017.
30.
Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes | SpringerLink. Accessed October 21, 2020. https://link.springer.com/arti....
31.
Rezaei NJ, Bazzazi AM, Alavi SAN. Neurotoxicity of the antibiotics: A comprehensive study. Neurology India. 2018; 66(6): 1732. doi: 10.4103/0028-3886.246258.
32.
Zareifopoulos N, Panayiotakopoulos G. Neuropsychiatric Effects of Antimicrobial Agents. Clin Drug Investig. 2017; 37(5): 423–437. doi: 10.1007/s40261-017-0498-z.
33.
Quinolone antibiotics and suicidal behavior: analysis of the World Health Organization’s adverse drug reactions database and discussion of potential mechanisms | SpringerLink. Accessed October 21, 2020. https://link.springer.com/arti....
34.
Lurie I, Yang Y-X, Haynes K, Mamtani R, Boursi B. Antibiotic Exposure and the Risk for Depression, Anxiety, or Psychosis: A Nested Case-Control Study. J Clin Psychiatry. 2015; 76(11): 1552–1528. doi: 10.4088/JCP.15m09961.
35.
Evidence for neurotoxicity associated with amoxicillin in juvenile rats – O Atli, U Demir-Ozkay, S Ilgin, TH Aydin, EN Akbulut, E Sener, 2016. Accessed October 21, 2020. https://journals.sagepub.com/d....
36.
Full article: Ciprofloxacin-induced neurotoxicity: evaluation of possible underlying mechanisms. Accessed October 21, 2020. https://www.tandfonline.com/do....
37.
Czarny P, Gałecki P, Sliwinski T. Stres oksydacyjny oraz uszkodzenia i naprawa DNA w zaburzeniach depresyjnych Oxidative stress, DNA damage and repair in depression disorders. Published online December 1, 2015. doi: 10.17393/fpn.2016.01.006.
38.
Kruszewska H, Zareba T, Tyski S. Examination of antimicrobial activity of selected non-antibiotic medicinal preparations. Acta poloniae pharmaceutica. 2012; 69: 1368–1371.
39.
Munoz-Bellido JL, Munoz-Criado S, Garcìa-Rodrìguez JA. Anti-microbial activity of psychotropic drugs: Selective serotonin reuptake inhibitors. International Journal of Antimicrobial Agents. 2000; 14(3): 177–180. doi: 10.1016/S0924-8579(99)00154-5.
40.
Effect of Antidepressants and Psychological Therapies in Irr...: Official journal of the American College of Gastroenterology | ACG. Accessed October 21, 2020. https://journals.lww.com/ajg/A....
41.
Macedo D, Filho AJMC, Soares de Sousa CN, et al. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. Journal of Affective Disorders. 2017; 208: 22–32. doi: 10.1016/j.jad.2016.09.012.
42.
Skonieczna-Żydecka K, Łoniewski I, Misera A, et al. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology. 2019; 236(5): 1491-1512. doi: 10.1007/s00213-018-5102-6.
43.
Pełka-Wysiecka J, Kaczmarczyk M, Bąba-Kubiś A, et al. Analysis of Gut Microbiota and Their Metabolic Potential in Patients with Schizophrenia Treated with Olanzapine: Results from a Six-Week Observational Prospective Cohort Study. Journal of Clinical Medicine. 2019; 8(10): 1605. doi: 10.3390/jcm8101605.
44.
Bahr SM, Tyler BC, Wooldridge N, et al. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Translational Psychiatry. 2015; 5(10): e652–e652. doi: 10.1038/tp.2015.135.
45.
Liśkiewicz P, Kaczmarczyk M, Misiak B, et al. Analysis of gut microbiota and intestinal integrit markers of inpatients with major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. Published online August 19, 2020: 110076. doi: 10.1016/j.pnpbp.2020.110076.
46.
Łoniewski I, Misera A, Skonieczna-Żydecka K, et al. Major Depressive Disorder and gut microbiota – Association not causation. A scoping review. Progress in Neuro-Psychopharmacology and Biological Psychiatry. Published online September 23, 2020: 110111. doi: 10.1016/j.pnpbp.2020.110111.
47.
Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015; 48: 258–264. doi: 10.1016/j.bbi.2015.04.003.
48.
Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. Accessed October 21, 2020. https://www.ncbi.nlm.nih.gov/p....
49.
Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2018; 84(Pt A): 50–55. doi: 10.1016/j.pnpbp.2018.02.007.
50.
Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009; 1: 6. doi: 10.1186/1757-4749-1- 6.
51.
Messaoudi M, Violle N, Bisson J-F, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011; 2(4): 256–261. doi: 10.4161/gmic.2.4.16108.
52.
Misiak B, Łoniewski I, Marlicz W, et al. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2020; 102: 109951. doi: 10.1016/j.pnpbp.2020.109951.
53.
Skonieczna-Żydecka K, Marlicz W, Misera A, Koulaouzidis A, Łoniewski I. Microbiome—The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. Journal of Clinical Medicine. 2018; 7(12): 521. doi: 10.3390/jcm7120521.
54.
Karakula-Juchnowicz H, Rog J, Juchnowicz D, et al. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): a 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr J. 2019; 18(1): 50. doi: 10.1186/s12937-019-0475-x.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.