Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Diagnostic values of trimethylamine (TMA) and trimethylamine N-oxide (TMAO) in the prediction of gestational diabetes mellitus – a systematic review and meta-analysis
1
Department of Clinical Research and Development, LUXMED Group, Poland
2
Department of Toxicology, Faculty of Pharmacy, Medical University, Gdańsk, Poland
3
Department of Gynaecology, University Hospital, Zurich, Switzerland
4
Department of Obstetrics, University Hospital, Zurich, Switzerland
5
Department of Transplantation Medicine, Nephrology and Internal Diseases, Medical University, Warsaw, Poland
6
Department of Medical Anthropology, Institute of Rural Health, Lublin, Poland
7
Henry JN Taub Department of Emergency Medicine, Baylor College of Medicine, United States
8
Institute of Medical Science, Collegium Medicum, The John Paul II Catholic University of Lublin, Poland
9
Department of Children’s Diabetology, School of Medicine, Katowice, Medical University of Silesia, Poland
Corresponding author
Lukasz Szarpak
Henry JN Taub Department of Emergency Medicine, Baylor College of Medicine, United States
Henry JN Taub Department of Emergency Medicine, Baylor College of Medicine, United States
Ann Agric Environ Med. 2025;32(1):111-115
KEYWORDS
TOPICS
Biological agents posing occupational risk in agriculture, forestry, food industry and wood industry and diseases caused by these agents (zoonoses, allergic and immunotoxic diseases)Health effects of chemical pollutants in agricultural areas , including occupational and non-occupational effects of agricultural chemicals (pesticides, fertilizers) and effects of industrial disposal (heavy metals, sulphur, etc.) contaminating the atmosphere, soil and waterExposure to physical hazards associated with the use of machinery in agriculture and forestry: noise, vibration, dustState of the health of rural communities depending on various factors: social factors, accessibility of medical care, etc.
ABSTRACT
Introduction and objective:
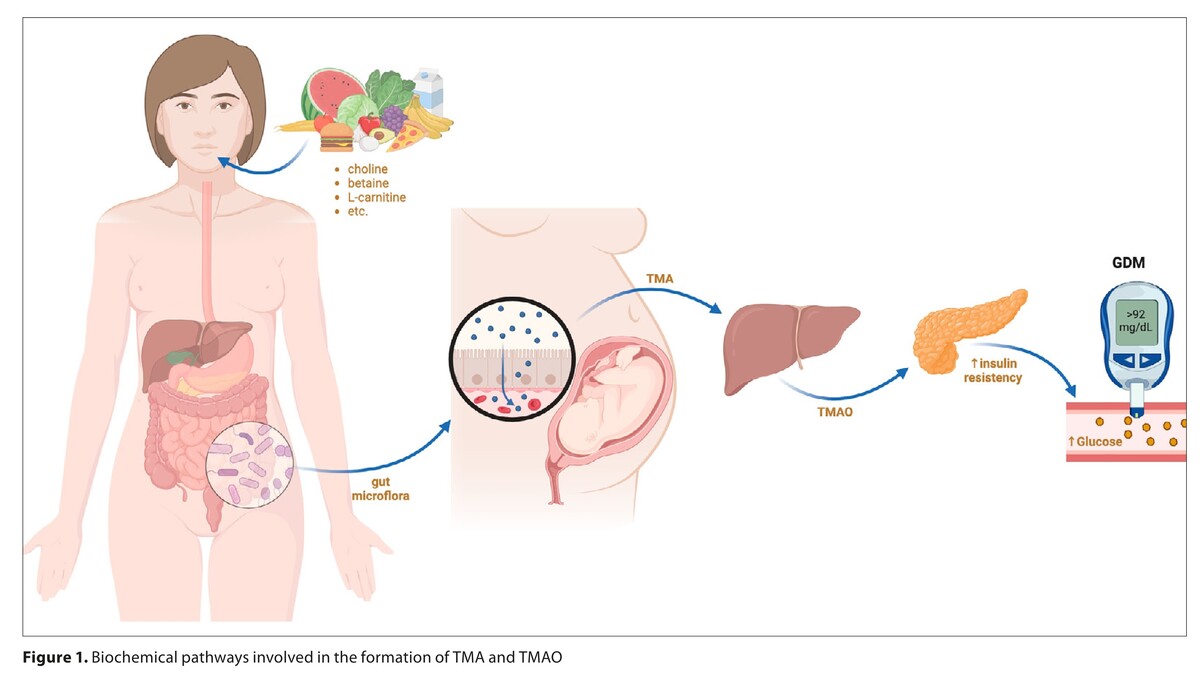
Gestational diabetes mellitus (GDM) is a growing concern for public health, affecting approximately 20% of pregnancies globally. This underscores an urgent need for improved diagnostic and management strategies. This study examines the relationship between trimethylamine N-oxide (TMAO) and its precursor trimethylamine (TMA) levels and GDM, aiming to deepen our understanding of GDM’s pathophysiology and identify novel therapeutic targets.
Material and methods:
The meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. The PubMed, Scopus, Web of Science, and the Cochrane Library electronic databases were comprehensively searched up to 11 July 2024.
Results:
The analysis included five studies, encompassing a total of 1,726 participants. The studies reported TMAO levels among GDM and non-GDM patients. The reported TMAO levels among GDM and non-GDM patients were 57.66 ± 42.2 and 47.94 ± 30.86, respectively (SMD = -0.49; 95%CI: -2.69 to 1.71; p = 0.66). However, TMA levels in the GDM group (224.28 ± 39.88) were statistically higher than in the non-GDM group (124.05 ± 21.93; SMD = 3.11; 95%CI: 2.84 to 3.37; p<0.001).
Conclusions:
The best available evidence indicates that while TMA levels are significantly increased in GDM, TMAO does not seem to have a diagnostic role in gestational diabetes mellitus. More prospective trials evaluating TMA and TMAO values among pregnant women with gestational diabetes mellitus are required.
Gestational diabetes mellitus (GDM) is a growing concern for public health, affecting approximately 20% of pregnancies globally. This underscores an urgent need for improved diagnostic and management strategies. This study examines the relationship between trimethylamine N-oxide (TMAO) and its precursor trimethylamine (TMA) levels and GDM, aiming to deepen our understanding of GDM’s pathophysiology and identify novel therapeutic targets.
Material and methods:
The meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines. The PubMed, Scopus, Web of Science, and the Cochrane Library electronic databases were comprehensively searched up to 11 July 2024.
Results:
The analysis included five studies, encompassing a total of 1,726 participants. The studies reported TMAO levels among GDM and non-GDM patients. The reported TMAO levels among GDM and non-GDM patients were 57.66 ± 42.2 and 47.94 ± 30.86, respectively (SMD = -0.49; 95%CI: -2.69 to 1.71; p = 0.66). However, TMA levels in the GDM group (224.28 ± 39.88) were statistically higher than in the non-GDM group (124.05 ± 21.93; SMD = 3.11; 95%CI: 2.84 to 3.37; p<0.001).
Conclusions:
The best available evidence indicates that while TMA levels are significantly increased in GDM, TMAO does not seem to have a diagnostic role in gestational diabetes mellitus. More prospective trials evaluating TMA and TMAO values among pregnant women with gestational diabetes mellitus are required.
REFERENCES (25)
1.
American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44:S15–33. doi:10.2337/dc21-ad09.
2.
Wang H, Li N, Chivese T, Werfalli M, et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res Clin Pract. 2022;183. doi:j.diabres.2021.109050.
3.
Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59:1396–1399. doi:10.1007/s00125-016-3985-5.
4.
Sparks JR, Ghildayal N, Hivert MF, Redman LM. Lifestyle interventions in pregnancy targeting GDM prevention: looking ahead to precision medicine. Diabetol. 2022;65:1814–1824. doi:10.1007/s00125-022-05658-w.
5.
ElSayed NA, Aleppo G, Aroda VR, et al. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S254-S266. doi:10.2337/dc23-S015.
6.
Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine n-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 2018;10. doi:10.3390/nu10101398.
7.
Tang WHW, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res2014;116:448–455. doi:10.1161/CIRCRESAHA.116.305360.
8.
Huo X, Li J, Cao YF, et al. Trimethylamine N-Oxide Metabolites in Early Pregnancy and Risk of Gestational Diabetes: A Nested Case-Control Study. J Clin Endocrinol Metab. 2019;104(11):5529–5539. doi:10.1210/jc.2019-00710.
9.
Li P, Zhong C, Li S, et al. Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am J Clin Nutr. 2018;108(3):603–610. doi:10.1093/ajcn/nqy116.
10.
McArthur KL, Zhang M, Hong X, et al. Trimethylamine N-Oxide and Its Precursors Are Associated with Gestational Diabetes Mellitus and Pre-Eclampsia in the Boston Birth Cohort. Curr Dev Nutr. 2022;6. doi:10.1093/cdn/nzac108.
11.
Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–65. doi:10.1038/nature09922.
12.
Canyelles M, Tondo M, Cedó L, Farràs M, Escolà-Gil JC, Blanco-Vaca F. Trimethylamine N-oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int J Mol Sci. 2018;19. doi:10.3390/ijms19103228.
13.
Homayouni A, Bagheri N, Mohammad-Alizadeh-Charandabi S, et al. Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr Diabetes Rev. 2019;16:538–45. doi:10.2174/1573399815666190712193828.
14.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
15.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi:10.1007/s10654-010-9491-z.
16.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi:10.1186/1471-2288-5-13.
17.
Gao Y, Chen H, Li J, Ren S, Yang Z, Zhou Y, Xuan R. Alterations of gut microbiota-derived metabolites in gestational diabetes mellitus and clinical significance. J Clin Lab Anal. 2022;36(4):e24333. doi:10.1002/jcla.24333.
18.
Gong X, Du Y, Li X, Yang J, Zhang X, Wei Y, Zhao Y. Maternal Plasma Betaine in Middle Pregnancy Was Associated with Decreased Risk of GDM in Twin Pregnancy: A Cohort Study. Diabetes Metab Syndr Obes. 2021 Jun 3;14:2495–2504. doi:10.2147/DMSO.S312334.
19.
Spanou L, Dimou A, Kostara CE, Bairaktari E, Anastasiou E, Tsimihodimos V. A Study of the Metabolic Pathways Affected by Gestational Diabetes Mellitus: Comparison with Type 2 Diabetes. Diagnostics. 2022;12:2881. doi:10.3390/diagnostics12112881.
20.
Lorenzo-Almorós A, Hang T, Peiró C, et al. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc Diabetol. 2019;18(1):140. doi:10.1186/s12933-019-0935-9.
21.
Yuan XS, Shi H, Wang HY, Yu B, Jiang J. Ficolin-3/adiponectin ratio for the prediction of gestational diabetes mellitus in pregnant women. J Diabetes Investig. 2018;9(2):403–410. doi:10.1111/jdi.12688.
22.
Wander PL, Boyko EJ, Hevner K, et al. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res Clin Pract. 2017;132:1–9. doi:10.1016/j.diabres.2017.07.024.
23.
Shaarbaf Eidgahi E, Nasiri M, Kariman N, et al. Diagnostic accuracy of first and early second trimester multiple biomarkers for prediction of gestational diabetes mellitus: a multivariate longitudinal approach. BMC Pregnancy Childbirth. 2022;22(1):13. doi:10.1186/s12884-021-04348-6.
24.
Wu K, Cheng Y, Li T, et al. The utility of HbA1c combined with haematocrit for early screening of gestational diabetes mellitus. Diabetol Metab Syndr. 2018;10:14. doi:10.1186/s13098-018-0314-9.
25.
Ruszała M, Niebrzydowska M, Pilszyk A, Kimber-Trojnar Ż, Trojnar M, Leszczyńska-Gorzelak B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus. Int J Mol Sci. 2021;22(21):11578. doi:10.3390/ijms222111578.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.