Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Contribution of tick-borne diseases to mortality in juvenile free-living cervids
1
Department of Eco-Epidemiology of Parasitic Diseases, Institute of Developmental Biology and Biomedical Sciences, Faculty of Biology, University of Warsaw, Poland
Corresponding author
Ann Agric Environ Med. 2022;29(2):215-219
KEYWORDS
TOPICS
Biological agents posing occupational risk in agriculture, forestry, food industry and wood industry and diseases caused by these agents (zoonoses, allergic and immunotoxic diseases)Prevention of occupational diseases in agriculture, forestry, food industry and wood industry
ABSTRACT
Introduction and objective:
Reports on tick-borne infections in free-living juvenile animals and their impact on survival of cervids in nature are lacking. The aim of the study was to detect and identify the Babesia and Anaplasma phagocytophilum species/ecotypes that may have contributed to the death of juvenile animals from a wildlife rescue centre in spring 2020.
Material and methods:
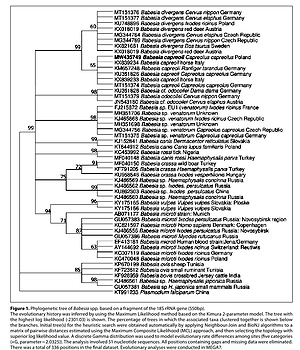
PCR amplification and sequencing of two genetic markers (18S rDNA and cox1 for Babesia, 16S rDNA and groEL for A. phagocytophilum) were used for screening eleven samples derived from juvenile animals which died in a rescue centre (seven roe deer Capreolus capreolus, one elk Alces alces, one red squirrel Sciurus vulgaris, one European beaver Castor fiber, one red fox Vulpes vulpes). Phylogenetic analysis of full-length 18S rDNA sequence was performed to enable differentiation between two closely-related species infecting wild ungulates, Babesia capreoli and Babesia divergens (zoonotic).
Results:
The occurrence of the typical SNPs of B. capreoli at two discriminating positions in the 18S rRNA gene allowed identification of B. capreoli infection in a roe deer calf. In two calves, Anaplasma phagocytophilum ecotype 2 was identified, including the same calf co-infection. No Babesia DNA was amplified in an elk calf treated for babesiosis. Splenomegaly was recorded in roe deer calves with A. phagocytophilum and A. phagocytophilum + B. capreoli infection.
Conclusions:
The study revealed that tick-borne infections are common among juvenile, free-living cervids and may contribute to their mortality.
Reports on tick-borne infections in free-living juvenile animals and their impact on survival of cervids in nature are lacking. The aim of the study was to detect and identify the Babesia and Anaplasma phagocytophilum species/ecotypes that may have contributed to the death of juvenile animals from a wildlife rescue centre in spring 2020.
Material and methods:
PCR amplification and sequencing of two genetic markers (18S rDNA and cox1 for Babesia, 16S rDNA and groEL for A. phagocytophilum) were used for screening eleven samples derived from juvenile animals which died in a rescue centre (seven roe deer Capreolus capreolus, one elk Alces alces, one red squirrel Sciurus vulgaris, one European beaver Castor fiber, one red fox Vulpes vulpes). Phylogenetic analysis of full-length 18S rDNA sequence was performed to enable differentiation between two closely-related species infecting wild ungulates, Babesia capreoli and Babesia divergens (zoonotic).
Results:
The occurrence of the typical SNPs of B. capreoli at two discriminating positions in the 18S rRNA gene allowed identification of B. capreoli infection in a roe deer calf. In two calves, Anaplasma phagocytophilum ecotype 2 was identified, including the same calf co-infection. No Babesia DNA was amplified in an elk calf treated for babesiosis. Splenomegaly was recorded in roe deer calves with A. phagocytophilum and A. phagocytophilum + B. capreoli infection.
Conclusions:
The study revealed that tick-borne infections are common among juvenile, free-living cervids and may contribute to their mortality.
ACKNOWLEDGEMENTS
The authors express their thanks to Professor Jerzy M. Behnke
at the University of Nottingham, UK, for the critical review
and linguistic proofreading of this article.
FUNDING
Ethics approval and consent to participate: not applicable.
Consent for publication: not applicable Availability of data
and materials: all data generated or analysed during this
study are included in this published article. Competing
interests: the authors declare that they have no competing
interests. Funding: the study was supported by the National
Science Centre (NCN) Sonata Bis grant no. 2014/14/E/
NZ7/00153 (AB). Authors’ contributions: AB conceived the
study and prepared ms; AB, MK, MA and DDS performed the
sampling and laboratory analyses. DDS and MK performed
phylogenetic analyses.
REFERENCES (36)
1.
Rosef O, Paulauskas A, Radzijevskaja J. Prevalence of Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in questing Ixodes ricinus ticks in relation to the density of wild cervids. Acta Vet Scand. 2009; 27: 47. doi: 10.1186/1751-0147-51-47.
2.
Medlock JM, Hansford KM, Bormane A, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors. 2013; 6: 1.
3.
Borowik T, Cornulier T, Jędrzejewska B. Environmental factors shaping ungulate abundances in Poland. Acta Theriol. 2013; 58: 403–413.
4.
Orłowska L, Rembacz W. Population dynamics and structure of roe deer (Capreolus capreolus) inhabiting small-size forests in north-western Poland. Folia Zool. 2016: 6552–6558.
5.
Panek M. Sytuacja zwierzyny łownej w Polsce – wyniki monitoringu 2019. Stacja Badawcza PZŁ Czempiń. 2020: 3–4. (in Polish).
6.
Raczynski J, Ratkiewicz M. Funkcjonowanie populacji łosia w Polsce. Ann Warsaw Univ Life Sci – SGGW. 2011; 50: 51–56. (in Polish).
7.
Borowik T, Ratkiewicz M, Maślanko W, Duda N, et al. Living on the edge – the predicted impact of renewed hunting on moose in national parks in Poland. Basic Appl Ecol. 2018; 30: 87–95.
8.
Hodžić A, Alić A, Fuehrer HP, et al. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors. 2015; 8: 88.
9.
Mierzejewska EJ, Dwużnik D, Koczwarska J, et al. The red fox (Vulpes vulpes), a possible reservoir of Babesia vulpes, B. canis and Hepatozoon canis and its association with the tick Dermacentor reticulatus occurrence. Ticks Tick Borne Dis. 2021; 12: 101551.
10.
Bonnet S, Jouglin M, L’Hostis M, et al. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg Infect Dis. 2007; 13: 1208–1210. doi: 10.3201/eid1308.061560.
11.
Bonnet S, Jouglin M, Malandrin L, et al. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitol. 2007; 134: 197–207. doi: 10.1017/S0031182006001545.
12.
Oosthuizen MC, Zweygarth E, Collins NE, et al. Identification of a novel Babesia sp. from a sable antelope (Hippotragus niger Harris, 1838). J Clin Microbiol. 2008; 46: 2247–2251.
13.
Matjila PT, Leisewitz AL, Oosthuizen MC, et al. Detection of a Theileria species in dogs in South Africa. Vet Parasitol. 2008; 157: 34–40.
14.
Malandrin L, Jouglin M, Sun Y, et al. Redescription of Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer ( Capreolus capreolus): isolation, cultivation, host specificity, molecular characterisation and differentiation from Babesia divergens. Int J Parasitol. 2010; 40: 277–284.
15.
Kowalec M, Szewczyk T, Welc-Falęciak R, et al. Ticks and the city – are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit Vectors. 2017; 10: 573.
16.
Kowalec M, Szewczyk T, Welc-Falęciak R, et al. Rickettsiales occurrence and co-occurrence in Ixodes ricinus ticks in natural and urban areas. Microb Ecol. 2019; 77: 890–904. doi: 10.1007/s00248-018-1269-y.
17.
Alberti A, Zobba R, Chessa B, A et al. Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl Environ Microbiol. 2005; 71: 6418–6422. doi: 10.1128/AEM.71.10.6418-6422.2005.
18.
Katoh K, Misawa K, Kuma K, et al. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002; 30: 3059–3066.
19.
Jahfari S, Coipan EC, Fonville M, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014; 7: 365.
20.
Trifinopoulos J, Nguyen LT, von Haeseler A, et al. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016; 8: W232–235.
21.
Katargina O, Geller J, Alekseev A, et al. Identification of Anaplasma phagocytophilum in tick populations in Estonia, the European part of Russia and Belarus. Clin Microbiol Infect. 2012; 18: 40–46.
22.
Welc-Faleciak R, Bajer A, Bednarska M, et al. Long term monitoring of Babesia microti infection in BALB-c mice using nested PCR. Ann Agric Environ Med. 2007; 14: 287–290.
24.
Welc-Falęciak R, Werszko J, Cydzik K, et al. Co-infection and genetic diversity of tick-borne pathogens in roe deer from Poland. Vector Borne Zoonotic Dis. 2013; 13: 277–288.
25.
Michel AO, Mathis A, Ryser-Degiorgis MP. Babesia spp. in European wild ruminant species: parasite diversity and risk factors for infection. Vet Res. 2014; 45: 1–11.
26.
Silaghi C, Fröhlich J, Reindl H, et al. Anaplasma phagocytophilum and Babesia species of sympatric roe deer (Capreolus capreolus), fallow deer (Dama dama), sika deer (Cervus nippon) and red deer (Cervus elaphus) in Germany. Pathogens. 2020; 9: 968.
27.
Azagi T, Jaarsma RI, Docters van Leeuwen A, et al. Circulation of Babesia Species and their exposure to humans through Ixodes ricinus. Pathogens. 2021; 10: 386. doi: org/10.3390/pathogens10040386.
28.
Dwużnik-Szarek D, Mierzejewska EJ, Rodo A, et al. Monitoring of expansion of Dermacentor reticulatus and occurrence ofcanine babesiosis in Poland in 2016–2018. Parasit Vectors. 2021; 14: 267. doi: org/10.1186/s13071-021-04758-7.
29.
Bajer A, Mierzejewska EJ, Rodo A, et al. The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland: Part 1: A population study on sled dogs during the racing season. Vet Parasitol. 2014; 202: 276–286. doi: 10.1016/j.vetpar.2013.12.033.
30.
Bajer A, Mierzejewska EJ, Rodo A, et al. The risk of vector-borneinfections in sled dogs associated with existing and new endemic areas in Poland. Part 2: Occurrence and control of babesiosis in a sled dog kennel during a 13-year-long period. Vet Parasitol. 2014; 202: 234–240.
31.
Jedrzejewski W, Schmidt K, Theuerkauf J, et al. Kill rates and predation by wolves on ungulate populations in Bialowieza Primeval Forest (Poland). Ecology. 2002; 83: 1341–1356.
32.
Dziki-Michalska K, Tajchman K, Budzyńska M. Increase in the moose (Alces alces L. 1758) population size in Poland: causes and consequences. Ann Warsaw Univ Life Sci – SGGW. 2019; 58: 201–214.
33.
Borowik T, Ratkiewicz M, Maślanko W, et al. 2020. The level of habitat patchiness influences movement strategy of moose in Eastern Poland. PLoS ONE 15: e0230521.
34.
Malmsten J, Dalin AM, Moutailler S, et al. Vector-borne zoonotic pathogens in Eurasian moose (Alces alces alces). Vector Borne Zoonotic Dis. 2019; 19: 207–211.
35.
Puraite I, Rosef O, Radzijevskaja J, et al. The first detection of species of Babesia Starcovici, 1893 in moose, Alces alces (Linnaeus), in Norway. Folia Parasitol (Praha). 2016; 63: 1–5.
36.
Matei IA, Estrada-Pena A, Cutler SJ, et al. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors. 2019; 12: 599. doi.org/10.1186/s13071-019-3852-6.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.