Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Bacteriological profile of the nasopharynx in patients with type 2 diabetes
1
Department of Family Medicine, School of Medicine with Division of Dentistry in Zabrze, Medical University of Silesia, Katowice, Poland
2
'Sanprom’ Family Doctors’ Practice, Zabrze, Poland
3
Diseases Clinic, Department of Internal Medicine, Diabetology and Nephrology / Faculty of Internal Medicine,
Diabetology and Nephrology, Zabrze, Poland
4
Central Laboratory, Clinical Hospital, Katowice, Poland
5
University of Strategic Planning, Dąbrowa Górnicza, Silesia, Poland
Corresponding author
Elżbieta Mizgała-Izworska
Department of Family Medicine, School of Medicine with the Division of Dentistry in Zabrze, Medical University of Silesia, Katowice, Poland, Department of Family Medicine, 41-800, Zabrze, Poland
Department of Family Medicine, School of Medicine with the Division of Dentistry in Zabrze, Medical University of Silesia, Katowice, Poland, Department of Family Medicine, 41-800, Zabrze, Poland
Ann Agric Environ Med. 2023;30(1):183-189
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Caring for people with diabetes is a challenge for doctors. GPs should be diagnostically vigilant and pay attention even to unusual symptoms reported by the patient, as they can progress quickly, impeding effective treatment. Targeted treatment of the bacteriological infection improves the prognosis in this group of patients. Its condition is to perform bacteriological tests. Statistics show that the infectious flora differ between people with diabetes and the general population.
Objective:
The aim of the study was to evaluate in a group of patients with type 2 diabetes without symptoms of active infection, the following: 1) composition of microflora in the nasal cavity and throat, with particular emphasis on the frequency and type of opportunistic and pathogenic microorganisms; 2) carrier status of Staphylococcus aureus bacteria in the nose, and its relationship to diabetes control/ other comorbidities predisposing to immuno-suppression.
Material and methods:
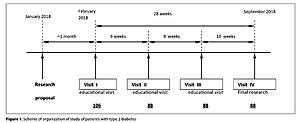
The study included 88 patients diagnosed with type 2 diabetes who were interviewed in the form of a questionnaire. Patients with additional systemic diseases and taking antibiotics within the last 6 weeks were excluded from the study. Microbiological tests required the collection of nasal and throat swabs from all enrolled patients.
Results:
The bacteriological analysis included 176 nasal and throat swabs taken from 88 patients with type 2 diabetes. A total of 627 species of microorganisms were identified, and 90 potentially pathogenic strains present in the nasal cavity and throat of the subjects were isolated and identified.
Conclusions:
People with type 2 diabetes who do not show symptoms of infection are often carriers of potentially pathogenic bacteria in the nasopharynx.
Caring for people with diabetes is a challenge for doctors. GPs should be diagnostically vigilant and pay attention even to unusual symptoms reported by the patient, as they can progress quickly, impeding effective treatment. Targeted treatment of the bacteriological infection improves the prognosis in this group of patients. Its condition is to perform bacteriological tests. Statistics show that the infectious flora differ between people with diabetes and the general population.
Objective:
The aim of the study was to evaluate in a group of patients with type 2 diabetes without symptoms of active infection, the following: 1) composition of microflora in the nasal cavity and throat, with particular emphasis on the frequency and type of opportunistic and pathogenic microorganisms; 2) carrier status of Staphylococcus aureus bacteria in the nose, and its relationship to diabetes control/ other comorbidities predisposing to immuno-suppression.
Material and methods:
The study included 88 patients diagnosed with type 2 diabetes who were interviewed in the form of a questionnaire. Patients with additional systemic diseases and taking antibiotics within the last 6 weeks were excluded from the study. Microbiological tests required the collection of nasal and throat swabs from all enrolled patients.
Results:
The bacteriological analysis included 176 nasal and throat swabs taken from 88 patients with type 2 diabetes. A total of 627 species of microorganisms were identified, and 90 potentially pathogenic strains present in the nasal cavity and throat of the subjects were isolated and identified.
Conclusions:
People with type 2 diabetes who do not show symptoms of infection are often carriers of potentially pathogenic bacteria in the nasopharynx.
FUNDING
This study was supported by the participating GP
cooperatives.
REFERENCES (23)
1.
Malinowska M, Tokarz-Deptuła B, Deptuła W. Mikrobiom człowieka. Post Mikrobol. 2017; 56: 33–42.
2.
Tokarz-Deptuła B, Śliwa-Dominiak J, Adamiak M, et al. Bakterie komensalne a odporność układu pokarmowego, oddechowego i moczowo-płciowego. Postępy Hig Med Dosw. 2016; 70: 599–609.
3.
Chwalba A, Otto-Buczkowska E. Participation of the microbiome in the pathogenesis of diabetes mellitus. Clin Diabetol. 2017; 6: 178–181.
4.
Pokrzywnicka P, Gumprecht J. Intestinal microbiota and its relationship with diabetes and obesity. Clin Diabetol. 2016; 5: 164–172.
5.
Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019 Nov 7;17(1):87. doi:.
6.
Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now?. Diabetes Res Clin Pract. 2018;146:111–118.
7.
Malinowska M, Tokarz-Deptuła B, Wiesław Deptuła W. Mikrobiom układu oddechowego w warunkach fizjologicznych i patologicznych. Post Mikrobiol. 2016; 55: 279–283.
8.
Schenck LP, Surette MG, Bowdish DM. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590(21):3705–3720.
9.
Sulikowska A. Nosicielstwo nosogardłowe wybranych patogenów bakteryjnych: Streptococcus pneumoniae, Haemophilus infuenzae i Moraxella catarrhalis. Nowa Med. 2009; 2: 124–130.
10.
Krishnan K, Chen T, Paster BJ. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017;23:276–286.
11.
Long J, Cai Q, Steinwandel M, et al. Association of oral microbiome with type 2 diabetes risk. J Periodontal Res. 2017;52:636–643.
12.
Pekuz S, Soysal A, Akkoc G, et al. Prevalence of Nasopharyngeal Carriage, Serotype Distribution, and Antimicrobial Resistance of Streptococcus pneumoniae among Children with Chronic Diseases. Jpn J Infect Dis. 2019; 72:7–13.
13.
Thaiss CA, Levy M, Grosheva I, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018 Mar 23;359(6382):1376–1383. doi: 10.1126/science.aar3318. Epub 2018 Mar 8. PMID: 29519916.
14.
Verhulst MJL, Loos BG, Gerdes VEA, et al. Evaluating All Potential Oral Complications of Diabetes Mellitus. Front Endocrinol (Lausanne). 2019 Feb 18;10:56. doi: 10.3389/fendo.2019.00056. PMID: 30962800; PMCID: PMC6439528.
15.
Alexiewicz JM, Kumar D, Smogorzewski M, et al. Polymorphonuclear leukocytes in non–insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann Intern Med. 1995; 123: 919–924.
16.
Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012; 16: S27-S36.
17.
Chang CH, Wang JL, Wu LC, et al. Diabetes, Glycemic Control, and Risk of Infection Morbidity and Mortality: A Cohort Study. Open Forum Infect Dis. 2019;6:ofz358.
18.
Fernández RDV, Díaz A, Bongiovanni B, et al. Evidence for a More Disrupted Immune-Endocrine Relation and Cortisol Immunologic Influences in the Context of Tuberculosis and Type 2 Diabetes Comorbidity. Front Endocrinol (Lausanne). 2020 Mar 20;11:126. doi: 10.3389/fendo.2020.00126. PMID: 32265833; PMCID: PMC7099637.
19.
Boyko EJ, Lipsky BA, Sandoval R, et al. NIDDM and prevalence of nasal Staphylococcus aureus colonization. San Luis Valley Diabetes Study. Diabetes Care. 1989 Mar;12(3):189–92. doi: 10.2337/diacare.12.3.189. PMID: 2702909.
20.
Essigmann HT, Hanis CL, DeSantis SM, et al. Worsening Glycemia Increases the Odds of Intermittent but Not Persistent Staphylococcus aureus Nasal Carriage in Two Cohorts of Mexican American Adults. Microbiol Spectr. 2022;10(3):e0000922. doi:10.1128/spectrum.00009–22.
21.
Wertheim HFL, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. The Lancet. 2004; 364: 703–705.
22.
Chikako Suto, Masahiro Morinaga, Tomoko Yagi, et al. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infection and Drug Resistance. 2012;5:37–41.
23.
Smit J, Søgaard M, Schønheyder H, et al. Diabetes and risk of community-acquired Staphylococcus aureus bacteremia: a population-based case-control study. Eur J Endocrinol. 2016; 174(5): 631–639.
Share
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.