Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Assessment of the functional return of an injured limb in a mouse model with Bcl-2 gene absent, following the application of locomotor training
1
Department of Adapted Physical Activity and Sport, Faculty of Health Sciences, Katowice, Poland
2
Faculty of Health Sciences in Katowice, Department of Sports Medicine and Physiology of Physical Exercise, Medical University of Silesia, Katowice, Poland
Corresponding author
Dariusz Górka
Faculty of Health Sciences in Katowice, Department of Sports Medicine and Physiology of Physical Exercise, Medical University of Silesia in Katowice Poland
Faculty of Health Sciences in Katowice, Department of Sports Medicine and Physiology of Physical Exercise, Medical University of Silesia in Katowice Poland
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
The impact of physical activity on human health is invaluable. Studies confirm an increase in neurotrophins in response to locomotor training. The Bcl-2 family of proteins, due to their diversity and pleiotropic actions, places damaged nerve cells on the path to apoptosis, making them a promising prospect in the treatment of neurodegenerative diseases. The aim of the experiment was to demonstrate the effect of the absence of the bcl-2 gene on the process of peripheral nerve regeneration in animals subjected to locomotor training.
Material and methods:
The first group consisted of animals with an absent bcl-2 gene, 129S1/SvImJBcl2tm1Mpin/J (N=40). Group E consisted of animals subjected to sciatic nerve damage with absent bcl-2 gene (N=20), which were subjected to locomotor training, while mice in which the sciatic nerve was cut (N=20) but no training programme was applied represent group K. The evaluation used an automatic gait analysis system for mice and rats from Nodus system Cat&Walk. The study analyzed footprints on a treadmill controlling the mechanism of locomotor movements for analysis before sciatic nerve damage and at 7, 14 and 28 days after treatment.
Results:
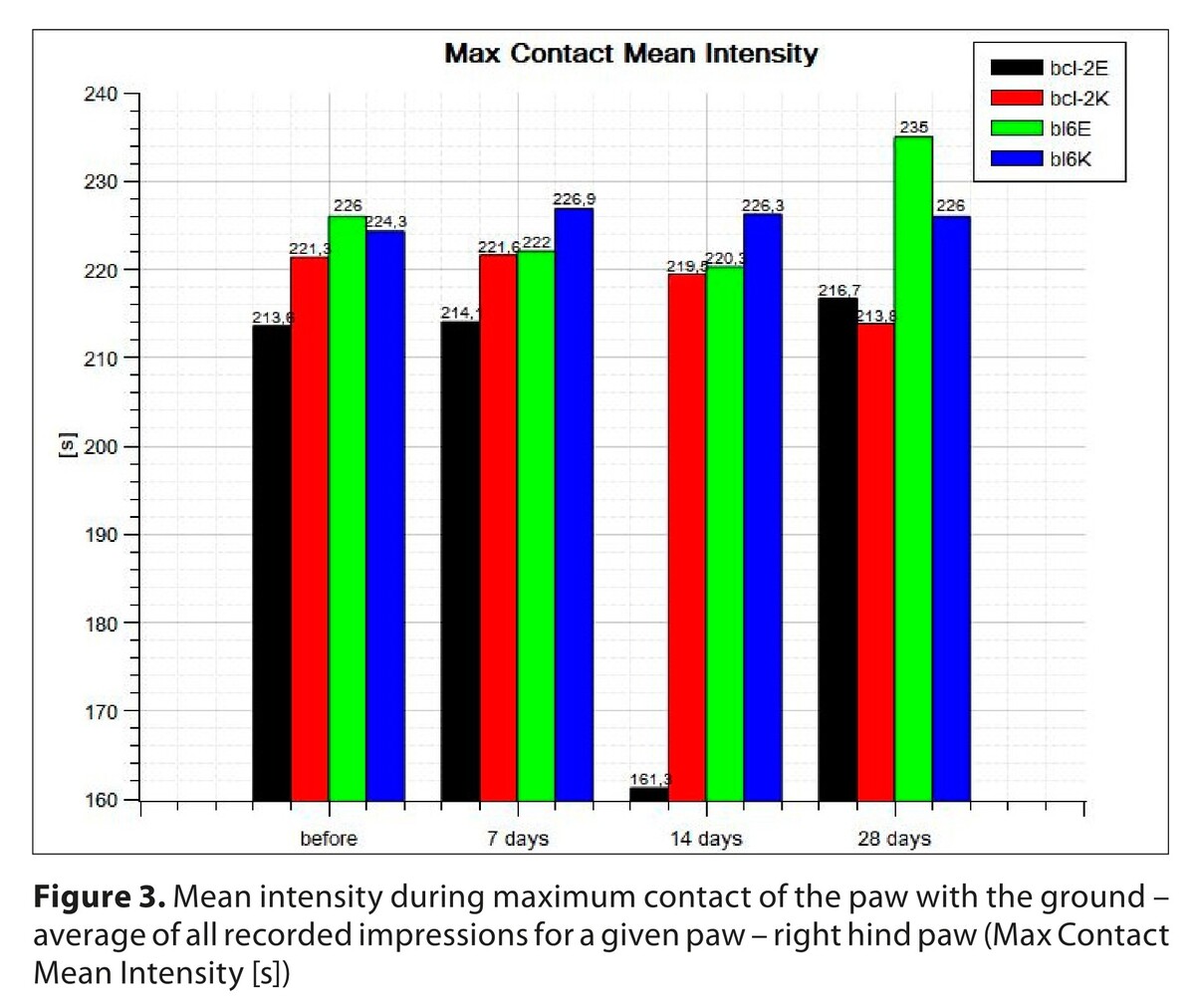
Both strains showed statistically significant differences in the experimental group compared to the untrained group, as well as a statistically significant difference in trained mice between the Bcl-2 and bl6 strains at day 14 after sciatic nerve injury, and at day 28 after sciatic nerve injury in the Bcl-2 group compared to the bl6 group, both subjected to training.
Conclusions:
Regeneration of the sciatic nerve in the locomotor-trained animals studied on the basis of the Cat&Walk study showed that significant improvement occurred in the group of Bcl-2 mice subjected to locomotor training on day 14 after nerve injury.
The impact of physical activity on human health is invaluable. Studies confirm an increase in neurotrophins in response to locomotor training. The Bcl-2 family of proteins, due to their diversity and pleiotropic actions, places damaged nerve cells on the path to apoptosis, making them a promising prospect in the treatment of neurodegenerative diseases. The aim of the experiment was to demonstrate the effect of the absence of the bcl-2 gene on the process of peripheral nerve regeneration in animals subjected to locomotor training.
Material and methods:
The first group consisted of animals with an absent bcl-2 gene, 129S1/SvImJBcl2tm1Mpin/J (N=40). Group E consisted of animals subjected to sciatic nerve damage with absent bcl-2 gene (N=20), which were subjected to locomotor training, while mice in which the sciatic nerve was cut (N=20) but no training programme was applied represent group K. The evaluation used an automatic gait analysis system for mice and rats from Nodus system Cat&Walk. The study analyzed footprints on a treadmill controlling the mechanism of locomotor movements for analysis before sciatic nerve damage and at 7, 14 and 28 days after treatment.
Results:
Both strains showed statistically significant differences in the experimental group compared to the untrained group, as well as a statistically significant difference in trained mice between the Bcl-2 and bl6 strains at day 14 after sciatic nerve injury, and at day 28 after sciatic nerve injury in the Bcl-2 group compared to the bl6 group, both subjected to training.
Conclusions:
Regeneration of the sciatic nerve in the locomotor-trained animals studied on the basis of the Cat&Walk study showed that significant improvement occurred in the group of Bcl-2 mice subjected to locomotor training on day 14 after nerve injury.
REFERENCES (25)
1.
Gieroba B. Wpływ aktywności fizycznej na zdrowie psychiczne i funkcje poznawcze. Med Og Nauk Zdr. 2019;25(3):153–161. https://doi.org/10.26444/monz/....
2.
Caponnetto P, Casu M, Amato M, et al. The Effects of Physical Exercise on Mental Health: From Cognitive Improvements to Risk of Addiction. Int J Environ Res Public Health. 2021;19;18(24):13384. https://doi.org/10.3390/ijerph....
3.
Valenzuela PL, Castillo-García A, Morales JS, et al. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res Rev. 2020;62:101108. 10.1016/j.arr.2020.101108.
4.
Sumińska S. Wpływ aktywności fizycznej na sprawność poznawczą. Med Pr. 2021;72(4):437–450. https://doi.org/10.13075/mp.58....
5.
Dyrla-Mularczyk K, Giemza-Urbanowicz W. Wpływ aktywności fizycznej na funkcjonowanie układu nerwowego i procesy poznawcze – przegląd badań. [The influence of physical activity on the functioning of the nervous system and cognitive processes – research review.] Neuropsychiatria i Neuropsychologia. 2019;14.3–4:84–91. https://doi.org/10.5114/nan.20....
6.
Murawska-Ciałowicz E, Wiatr M, Ciałowicz M, et al. BDNF Impact on Biological Markers of Depression-Role of Physical Exercise and Training. Int J Environ Res Public Health. 2021;15;18(14):7553. https://doi.org/10.3390/ijerph....
7.
Ashcroft SK, Ironside DD, Johnson L, et al. Effect of Exercise on Brain-Derived Neurotrophic Factor in Stroke Survivors: A Systematic Review and Meta-Analysis. Stroke. 2022;53(12):3706–3716. https://doi.org/10.1161/STROKE....
8.
Xing Y, Bai Y. A Review of Exercise-Induced Neuroplasticity in Ischemic Stroke: Pathology and Mechanisms. Mol Neurobiol. 2020;57(10):4218–4231. https://doi.org/10.1007/s12035....
9.
Vandamme Thierry F. Use of rodents as models of human diseases. J Pharmacy Bioallied Sci. 2014;6(1):2–9. https://doi.org/10.4103/0975-7....
10.
Jones TS, Holland EC. Animal models for glioma drug discovery. Expert Opin Drug Discov. 2011;6:1271–83. https://doi.org/10.1517/174604....
11.
Flisikowska T, Kind A, Schnieke A. The new pig on the block: Modelling cancer in pigs. Transgenic Res. 2013;22:673–80. https://doi.org/10.1007/s11248....
12.
Zhou XB, Liu N, Wang D, et al. Neuroprotective effect of ischemic postconditioning on sciatic nerve transection. Neural Regen Res. 2018;13:492–496. https://doi.org/10.4103/1673-5....
13.
Wang ZM, Dai CF, Kanoh N, et al. Apoptosis and expression of BCL-2 in facial motoneurons after facial nerve injury. Otol Neurotol. 2002; 23:397–404. https://doi.org/10.1097/001294....
14.
Maugeri G, D’Agata V, Trovato B, et al. The role of exercise on peripheral nerve regeneration: from animal model to clinical application. Heliyon. 2021;29;7(11):e08281. 10.1016/j.heliyon.2021.e08281.
15.
Maugeri G, D’Agata V, Magrì B, et al. Neuroprotective effects of physical activity via the adaptation of astrocytes. Cells. 2021;18;10(6):1542. https://doi.org/10.3390/cells1....
16.
Höke A, Brushart T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp Neurol. 2010;223(1):1–4. https://doi.org/10.1016/j.expn....
17.
Arbat-Plana A, Cobianchi S, Herrando-Grabulosa M, et al. Endogenous modulation of TrkB signaling by treadmill exercise after peripheral nerve injury. Neurosci. 2017;6;340:188–200. https://doi.org/ 10.1016/j.neuroscience.2016.10.057.
18.
O’Neill P, Lindsay SL, Pantiru A, et al. Sulfatase-mediated manipulation of the astrocyte-Schwann cell interface. Glia. 2016;65(1):19–33. https://doi.org/10.1002/glia.2....
19.
Kappos EA, Sieber PK, Engels PE, et al. Validity and reliability of the CatWalk system as a static and dynamic gait analysis tool for the assessment of functional nerve recovery in small animal models. Brain Behav. 2017;18;7(7):e00723. https://doi.org/10.1002/brb3.7....
20.
Isaev NK, Stelmashook EV, Genrikhs EE. Role of Nerve Growth Factor in Plasticity of Forebrain Cholinergic Neurons. Biochemistry. 2017;82(3):291–300. https://doi.org/10.1134/S00062....
21.
Sasi M, Vignoli B, Canossa M, Blum R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017;469(5–6):593–610. https://doi.org/10.1007/s00424....
22.
Hempstead BL. Brain-Derived Neurotrophic Factor: Three Ligands. Many Actions. Trans Am Clin Climatol Assoc. 2015;126:9–1.
23.
Molteni R, Zheng JQ, Ying Z, et al. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2014;1;101(22):8473–8. https://doi.org/10.1073/pnas.0....
24.
Gouveia D, Cardoso A, Carvalho C, et al. Early Intensive Neurorehabilitation in Traumatic Peripheral Nerve Injury-State of the Art. Animals (Basel). 2024 Mar 13;14(6):884. doi:10.3390/ani14060884.
25.
Wilson RJ, Drake JC, Cui D, et al. Voluntary running protects against neuromuscular dysfunction following hindlimb ischemia-reperfusion in mice. J Appl Physiol. (1985). 2019 Jan 1;126(1):193–201. doi:10.1152/japplphysiol.00358.2018. Epub 2018 Nov 15.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.