Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
CASE REPORT
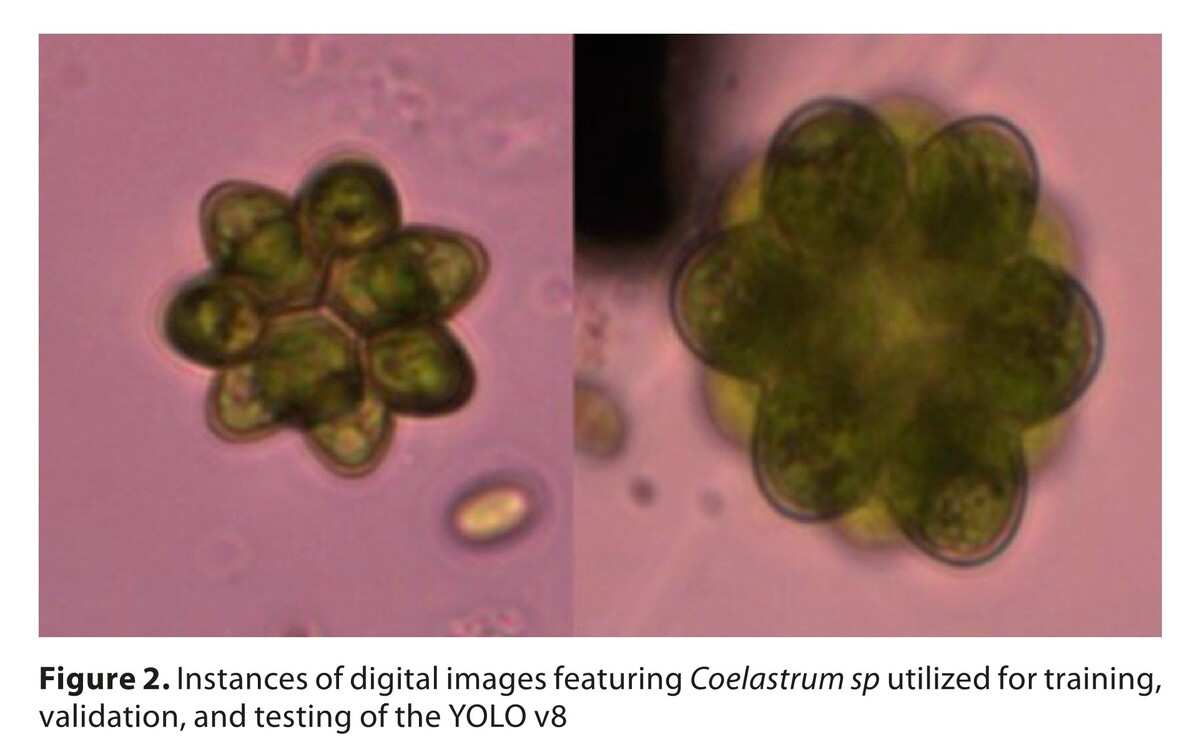
Application of automatic image analysis using a Deep Learning Neural Network for assessing the growth of green algae containing carotenoids – importance for environment, health and aquaculture
1
Department of Water Purification and Protection, Rzeszów University of Technology, Rzeszów, Poland
2
Department of Applied Mathematics, Faculty of Mathematics and Information Technology, Lublin University of Technology, Lublin, Poland
3
Department of Water Supply and Sewage Disposal, Lublin University of Technology, Lublin, Poland
These authors had equal contribution to this work
Corresponding author
Monika M. Zdeb
Department of Water Purification and Protection, Rzeszow University of Technology, al. Powstancow Warszawy 6, 35-959, Rzeszów, Poland
Department of Water Purification and Protection, Rzeszow University of Technology, al. Powstancow Warszawy 6, 35-959, Rzeszów, Poland
Ann Agric Environ Med. 2025;32(1):157-162
KEYWORDS
carotenoidsgreen algaeautomatic image analysisHaematococcusCoelastrumDeep Learning Neural Networkbioindicationaquaculture
TOPICS
ABSTRACT
Using deep learning and neural networks enables us to greatly speed-up quantitative studies and provide a useful tool for analyzing microscopic images. Studies conducted on selected algae Haematococcus and Coelastrum sp. confirm the feasibility of using the deep learning neural network. The confusion matrix demonstrated the numbers of errors generated by the YOLO v8 network in relation to the validation dataset. It indicated a higher number of errors in the detection of Haematococcus than Coleastrum. The F1 score, as the harmonic mean of precision and recall, is significantly higher for the class Coelastrum sp. than for Haematococcus sp. Machine learning can be applied not only to the detection of individual cells, but also to the detection of colonies over a wide range of sizes. This article discussed the technical and practical aspects of implementing these advanced methods and highlighted their importance in the aquaculture, food, medical, sustainable energy, and environmental sectors.
ACKNOWLEDGEMENTS
The research was carried out as part of the task commissioned under the title "Politechnical Network VIA CARPATIA named after the President of the Republic of Poland Lech Kaczyński" financed from the Special Purpose Grant of the Minister of Science, contract number MEiN/2022/DPI/2578 action "PO SĄSIEDZKU - inter-university research internships and study visits.
REFERENCES (25)
1.
Gamrat R, Puc M, Gałczyńska M, et al. Differences in the pollen content of varieties of Polish honey from urban and rural apiaries. Acta Univ Cibinien. Ser E: Food Technol. 2022;26(1):109–122. https://doi.org/10.2478/aucft-....
2.
Choi H-J, Lee S-M. Effects of microalgae on the removal of nutrients from wastewater: Various concentrations of Chlorella vulgaris. Environ Eng Res. 2012;17(S1):S3-S8. https://doi.org/10.4491/eer.20....
3.
Dębowski M, Zieliński M, Krzemieniewski M, et al. Możliwość namnażania biomasy glonów na bazie odcieku pochodzącego z odwadniania osadów pofermentacyjnych. Ann Set Environ Protect. 2013;15(2):1612–1623. ISSN 1506 218X1612-1622.
4.
Yang Y, Tang S, Chen JP. Carbon capture and utilization by algae with high concentration CO2 or bicarbonate as carbon source. Sci Total Environ. 2024;918:170325. https://doi.org/10.1016/j.scit....
5.
Iglina T, Iglin P, Pashchenko D. Industrial CO2 capture by algae: A review and recent advances. Sustainability. 2022;14:3801. https://doi.org/10.3390/su1407....
6.
Bhateria R, Dhaka R. Algae as biofuel. Biofuels. 2015;5(6):607–631. https://doi:10.1080/17597269.2....
7.
Neeti K, Gaurav K, Singh R. The potential of algae biofuel as a renewable and sustainable bioresource. Eng Proc. 2023;37(1):22. https://doi.org/10.3390/ECP202....
8.
Szczepocka E, Szulc B, Szulc K, et al. Diatom indices in the biological assessment of the water quality based on the example of a smalllowland river. Oceanol Hydrobiol Stud. 2014;43(3):265–273. https://doi.org/10.2478/s13545....
9.
Commission Implementing Regulation (EU) 2020/1820 of 2 December 2020 authorising the placing on the market of dried Euglena gracilis as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470.
10.
Le-Feuvre R, Moraga-Suazo P, Gonzalez J. Biotechnology applied to Haematococcus pluvialis Fotow: challenges and prospects for the enhancement of astaxanthin accumulation. J Appl Phycol. 2020;32:3831–3852 https://doi.org/10.1007/s10811....
11.
Thiyagarasaiyar K, Goh BH, Jeon YJ, et al. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar Drugs. 2020;18:323. https://doi.org/10.3390/md1806....
12.
Pogorzelska E, Hamułka J, Wawrzyniak A. Astaksantyna – budowa, właściwości i możliwości zastosowania w żywności funkcjonalnej. Żywność. Nauka. Technologia. Jakość. 2016;1(104):5–16. http://dx.doi.org/10.15193/znt....
13.
Ota S, Kawano S. Three-dimensional ultrastructure and hyperspectral imaging of metabolite accumulation and dynamics in Haematococcus and Chlorella. Microscopy. 2019;68(1):57–68. https://doi.org/10.1093/jmicro....
14.
Taye MM. Understanding of Machine Learning with Deep Learning: architectures, workflow, applications and future directions. Computers. 2023;12:91. https://doi.org/10.3390/comput....
15.
Picińska-Fałtynowicz J, Błachuta J. Klucz do identyfikacji organizmów fitoplanktonowych z rzek i jezior dla celów badań monitoringowych części wód powierzchniowych w Polsce. Warszawa: Główny Inspektorat Ochrony Środowiska; 2012;83. ISBN 978-83-61227-05-2, OCLC 839100228.
16.
Adam C, Haryono A. Morphological study of Coelastrum cambricum from the Peat Water of Palangka Raya, Indonesia. Nusantara Sci Technol Proceed. 2022;25:21–28. https://doi.org/10.11594/nstp.....
17.
Rauytanapanit M, Jancho K, Kusolkumbot P, et al. Nutrient deprivation-associated changes in green microalga Coelastrum sp. TISTR 9501RE enhanced potent antioxidant carotenoids. Mar Drugs. 2019;17:328. https://doi.org/10.3390/md1706....
18.
Staniszewski M, Dziadosz M, Zaburko J, et al. Automatic System for Acquisition and Analysis of Microscopic Digital Images containing activated sludge. Sci Technol Res J. 2024;18(7):51–61. https://doi.org/10.12913/22998....
19.
Dziadosz M, Majerek D, Łagód G. Microscopic studies of activated sludge supported by Automatic Image Analysis based on Deep Learning Neural Networks, J Ecol Eng. 2024;25(4):360–369. https://doi.org/10.12911/22998....
20.
Jamka K, Wróblewska-Łuczka P, Adamczuk P, et al. Methodology for preparing a cosmetic sample for the development of Microorganism Detection System (SDM) software and artificial intelligence learning to recognize specific microbial species. Ann Agric Environ Med. 2021;28(4):681–685. https://doi.org/10.26444/aaem/....
21.
Wu J, Xu W, He J, et al. YOLO for Penguin Detection and counting based on remote sensing images. Remote Sens. 2023;15:2598. https://doi.org/10.3390/rs1510....
22.
Codd GA, Morrison LF, Metcalf JS. Cyanobacterial toxins: Risk management for health protection. Toxicol Appl Pharmacol. 2005; 203(3):264–272. https://doi.org/10.1016/j.taap....
23.
Walo M, Majerek D, Kuzmina T, et al. Bioindication of surface water supported by automatic image analysis using Deep Learning Neural Network – Cyclotella Case Study. J Ecol Eng. 2024;25(12):366–375. https://doi.org/10.12911/22998....
24.
Yan H, Peng X, Chen Ch, et al. YOLOx model-based object detection for microalgal bioprocess. Algal Res. 2023;74:103178. https://doi.org/10.1016/j.alga....
25.
Oslan SNH, Shoparwe NF, Yusoff AH, et al. A Review on Haematococcus pluvialis bioprocess optimization of green and red stage culture conditions for the production of natural astaxanthin. Biomolecules. 2021;11(2):256. https://doi.org/10.3390/biom11....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.