Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Adult-onset Still’s disease in Poland – a nationwide population-based study
1
Department of Social Medicine and Public Health, Medical University, Warsaw, Poland
2
Department of Prevention of Environmental Hazards and Allergology, Medical University, Warsaw, Poland
3
National Institute of Public Health – National Institute of Hygiene, Warsaw, Poland
4
Institute of Rural Health, Lublin, Poland
Corresponding author
Ann Agric Environ Med. 2021;28(2):250-254
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Adult-onset Still’s disease (AOSD) is a rare systemic inflammatory disorder of unknown etiology, which affects young adults and is associated with multiple organ involvement and life-threatening complications. The aim of the study was to establish the incidence and prevalence of AOSD in Poland, and factors related to this disease among hospitalized patients.
Material and methods:
A retrospective, population-based study was conducted, using data from hospital discharge records compiled by the National Institute of Public Health in 2009–2018.
Results:
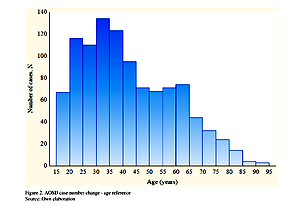
Based on hospitalization records and census data in a group of the 1,050 patients included in the study, women were predominant (60%) and patients’ mean and median ages at hospitalization were 42 (95% CI: 40.8–42.8) and 38 years, respectively. The average annual incidence rate of AOSD during the 10-year period was established at the level of 0.32/100,000 (95% CI: 0.30–0.34), and the point prevalence at the end of 2018 was 2.7/100,000. The most common comorbidities were: cardiovascular diseases (14%), diseases of the musculoskeletal system and connective tissue (14%), endocrine, nutritional and metabolic diseases (9%).
Conclusions:
AOSD is a rare disease Poland with gender and territorial differences in incidence ratek, and predominance of cardiovascular diseases among comorbidities. The presented data may be useful for comparisons of the Polish population with other geographical regions. Predominance of patients from urban regions and predominance of women may suggest environmental and personal factors in AOSD development; however, further research seems to be necessary.
Adult-onset Still’s disease (AOSD) is a rare systemic inflammatory disorder of unknown etiology, which affects young adults and is associated with multiple organ involvement and life-threatening complications. The aim of the study was to establish the incidence and prevalence of AOSD in Poland, and factors related to this disease among hospitalized patients.
Material and methods:
A retrospective, population-based study was conducted, using data from hospital discharge records compiled by the National Institute of Public Health in 2009–2018.
Results:
Based on hospitalization records and census data in a group of the 1,050 patients included in the study, women were predominant (60%) and patients’ mean and median ages at hospitalization were 42 (95% CI: 40.8–42.8) and 38 years, respectively. The average annual incidence rate of AOSD during the 10-year period was established at the level of 0.32/100,000 (95% CI: 0.30–0.34), and the point prevalence at the end of 2018 was 2.7/100,000. The most common comorbidities were: cardiovascular diseases (14%), diseases of the musculoskeletal system and connective tissue (14%), endocrine, nutritional and metabolic diseases (9%).
Conclusions:
AOSD is a rare disease Poland with gender and territorial differences in incidence ratek, and predominance of cardiovascular diseases among comorbidities. The presented data may be useful for comparisons of the Polish population with other geographical regions. Predominance of patients from urban regions and predominance of women may suggest environmental and personal factors in AOSD development; however, further research seems to be necessary.
REFERENCES (47)
1.
Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still’s disease. J Autoimmun. 2018; 93: 24–36.
2.
Nirmala N, Brachat A, Feist E, Blank N, Specker C, Witt M, et al. Geneexpression analysis of adult-onset Still’s disease and systemic juvenile idiopathic arthritis is consistent with a continuum of a single disease entity. Pediatr Rheumatol Online J. 2015; 13: 50.
3.
Vastert SJ, Jamilloux Y, Quartier P, Ohlman S, Osterling Koskinen L, Kullenberg T, et al. Anakinra in children and adults with Still’s disease. Rheumatology (Oxford). 2019; 58: vi9–22.
4.
Kadavath S, Efthimiou P. Adult-onset Still’s disease-pathogenesis, clinical manifestations, and new treatment options. Ann Med. 2015; 47: 6–14.
5.
Li H, Abramova I, Chesoni S, Yao Q. Molecular genetic analysis for periodic fever syndromes: a supplemental role for the diagnosis of adult-onset Still’s disease. Clin Rheumatol. 2018; 37: 2021–2026.
6.
Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992; 19: 424–430.
7.
Ahn SS, Yoo B-W, Jung SM, Lee S-W, Park Y-B, Song JJ. Application of the 2016 EULAR/ACR/PRINTO Classification Criteria for Macrophage Activation Syndrome in Patients with Adult-onset Still Disease. J Rheumatol. 2017; 44: 996–1003.
8.
Tada Y, Inokuchi S, Maruyama A, Suematsu R, Sakai M, Sadanaga Y, et al. Are the 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome applicable to patients with adult-onset Still’s disease? Rheumatol Int. 2019; 39: 97–104.
9.
Mavragani CP, Spyridakis EG, Koutsilieris M. Adult-Onset Still’s Disease: From Pathophysiology to Targeted Therapies. Int J Inflam. 2012; 2012: 879020.
10.
Fujita Y, Furukawa H, Asano T, Sato S, Yashiro Furuya M, Kobayashi H, et al. HLA-DQB1 DPB1 alleles in Japanese patients with adult-onset Still’s disease. Mod Rheumatol. 2019; 29: 843–847.
11.
Perez C, Artola V. Adult Still’s disease associated with Mycoplasma pneumoniae infection. Clin Infect Dis. 2001; 32: E105–106.
12.
Jia J, Shi H, Liu M, Liu T, Gu J, Wan L, et al. Cytomegalovirus Infection May Trigger Adult-Onset Still’s Disease Onset or Relapses. Front Immunol. 2019; 10: 898.
13.
Jamilloux Y, Gerfaud-Valentin M, Martinon F, Belot A, Henry T, Seve P. Pathogenesis of adult-onset Still’s disease: new insights from the juvenile counterpart. Immunol Res. 2015; 61: 53–62.
14.
Magadur-Joly G, Billaud E, Barrier JH, Pennec YL, Masson C, Renou P, et al. Epidemiology of adult Still’s disease: estimate of the incidence by a retrospective study in west France. Ann Rheum Dis. 1995; 54: 587–590.
15.
Wakai K, Ohta A, Tamakoshi A, Ohno Y, Kawamura T, Aoki R, et al. Estimated Prevalence and Incidence of Adult Still’s Disease: Findings by a Nationwide Epidemiological Survey in Japan. J Epidemiol. 1997; 7: 221–225.
16.
Evensen KJ, Nossent HC. Epidemiology and outcome of adult-onset Still’s disease in Northern Norway. Scand J Rheumatol. 2006; 35: 48–51.
17.
Cagatay Y, Gul A, Cagatay A, Kamali S, Karadeniz A, Inanc M, et al. Adult-onset Still’s disease. Int J Clin Pract. 2009; 63: 1050–1055.
18.
Mehta BY, Ibrahim S, Briggs W, Efthimiou P. Racial/Ethnic variations in morbidity and mortality in Adult Onset Still’s Disease: An analysis of national dataset. Semin Arthritis Rheum. 2019; 49: 469–473.
19.
Lenert A, Oh Gy, Ombrello MJ, Kim S. Clinical characteristics and comorbidities in adult-onset Still’s disease using a large US administrative claims database. Rheumatology (Oxford). 2020; 59: 1725–1733.
20.
Sfriso P, Priori R, Valesini G, Rossi S, Montecucco CM, D’Ascanio A, et al. Adult-onset Still’s disease: an Italian multicentre retrospective observational study of manifestations and treatments in 245 patients. Clin Rheumatol. 2016; 35: 1683–1689.
21.
Sakata N, Shimizu S, Hirano F, Fushimi K. Epidemiological study of adult-onset Still’s disease using a Japanese administrative database. Rheumatol Int. 2016; 36: 1399–1405.
22.
Mahfoudhi M, Shimi R, Turki S, Kheder A. Epidemiology and outcome of articular complications in adult onset Still’s disease. Pan Afr Med J. 2015; 22: 77.
23.
Franchini S, Dagna L, Salvo F, Aiello P, Baldissera E, Sabbadini MG. Adult onset Still’s disease: clinical presentation in a large cohort of Italian patients. Clin Exp Rheumatol. 2010; 28: 41–48.
24.
Suda T, Zoshima T, Takeji A, Suzuki Y, Mizushima I, Yamada K, et al. Elderly-onset Still’s Disease Complicated by Macrophage Activation Syndrome: A Case Report and Review of the Literature. Intern Med. 2020; 59: 721–728.
25.
Kim YJ, Koo BS, Kim Y-G, Lee C-K, Yoo B. Clinical features and prognosis in 82 patients with adult-onset Still’s disease. Clin Exp Rheumatol. 2014; 32: 28–33.
26.
Dudziec E, Pawlak-Buś K, Leszczyński P. Adult-onset Still’s disease as a mask of Hodgkin lymphoma. Reumatologia. 2015; 53: 106–110.
27.
Wawrzycki B, Krasowska D, Pietrzak A, Wielosz E, Majdan M, Lotti T. Urticarial rash, fever, and arthritis: A case of refractory Adult-onset Still’s disease with good response to tocilizumab. Dermatol Ther. 2019; 32: e13041.
28.
Bożek M, Konopko M, Wierzba-Bobrowicz T, Witkowski G, Makowicz G, Sienkiewicz-Jarosz H. Autoimmune meningitis and encephalitis in adult-onset still disease – Case report. Neurol Neurochir Pol. 2017; 51: 421–426.
29.
Kedzia A, Bołdys A, Krysiak R, Szkróbka W, Okopień B. Potential benefit of paracetamol administration in adult-onset Still’s disease. Pol Arch Med Wewn. 2009; 119: 595–598.
30.
Błasiak A, Błachowicz A, Gietka A, Rell-Bakalarska M, Franek E. Still’s disease in patient with silicone breast implants: case report. Pol Arch Med Wewn. 2008; 118: 65–67.
31.
Kanecki K, Nitsch-Osuch A, Gorynski P, Wierzba W, Tarka P, Tyszko P. Polyarteritis nodosa: decreasing incidence in Poland. Arch Med Sci. 2019; 15: 1308–1312.
32.
Batko B, Stajszczyk M, Świerkot J, Urbański K, Raciborski F, Jędrzejewski M, et al. Prevalence and clinical characteristics of rheumatoid arthritis in Poland: a nationwide study. Arch Med Sci. 2019; 15: 134–140.
33.
TIBCO Software Inc. (2017). Statistica (data analysis software system), version 13. http://statistica.io.
34.
Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011; 8: 1.
35.
Asanuma YF, Mimura T, Tsuboi H, Noma H, Miyoshi F, Yamamoto K, et al. Nationwide epidemiological survey of 169 patients with adult Still’s disease in Japan. Mod Rheumatol. 2015; 25: 393–400.
36.
Mehta BY, Ibrahim S, Briggs W, Efthimiou P. Racial/Ethnic variations in morbidity and mortality in Adult Onset Still’s Disease: An analysis of national dataset. Semin Arthritis Rheum. 2019; 49: 469–47.
37.
Yao HH, Wang YN, Zhang X, Zhao JX, Jia Y, Wang Z, et al. [Clinical characteristics and treatment outcomes of macrophage activation syndrome in adults: A case series of 67 patients]. [Article in Chinese] Beijing Da Xue Xue Bao Yi Xue Ban. 2019; 51: 996–1002.
38.
Gerfaud-Valentin M, Maucort-Boulch D, Hot A, Iwaz J, Ninet J, Durieu I, et al. Adult-onset still disease: manifestations, treatment, outcome, and prognostic factors in 57 patients. Medicine (Baltimore). 2014; 93: 91–99.
39.
Chen P-D, Yu S-L, Chen S, Weng X-H. Retrospective study of 61 patients with adult-onset Still’s disease admitted with fever of unknown origin in China. Clin Rheumatol. 2012; 31: 175–181.
40.
Kong X-D, Xu D, Zhang W, Zhao Y, Zeng X, Zhang F. Clinical features and prognosis in adult-onset Still’s disease: a study of 104 cases. Clin Rheumatol. 2010; 29: 1015–1019.
41.
Colina M, Zucchini W, Ciancio G, Orzincolo C, Trotta F, Govoni M. The evolution of adult-onset Still disease: an observational and comparative study in a cohort of 76 Italian patients. Semin Arthritis Rheum. 2011; 41: 279–285.
42.
Sun NZ, Brezinski EA, Berliner J, Haemel A, Connolly MK, Gensler L, et al. Updates in adult-onset Still disease: Atypical cutaneous manifestations and associations with delayed malignancy. J Am Acad Dermatol. 2015; 73: 294–303.
43.
Yilmaz S, Karakas A, Cinar M, Coskun O, Simsek I, Erdem H, et al. Adult onset Still’s disease as a paraneoplastic syndrome--a case report and review of the literature. Bull Hosp Jt Dis. 2013; 71: 156–60.
44.
Hofheinz K, Schett G, Manger B. Adult onset Still’s disease associated with malignancy-Cause or coincidence? Semin Arthritis Rheum. 2016; 45: 621–626.
45.
Liozon E, Ly KH, Vidal-Cathala E, Fauchais A-L. [Adult-onset Still’s disease as a manifestation of malignancy: report of a patient with melanoma and literature review]. [Article in French] Rev Med Interne. 2014; 35: 60–64.
46.
Maria ATJ, Le Quellec A, Jorgensen C, Touitou I, Riviere S, Guilpain P. Adult onset Still’s disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun Rev. 2014; 13: 1149–1159.
47.
Gerfaud-Valentin M, Seve P, Hot A, Broussolle C, Jamilloux Y. Pathophysiology, subtypes, and treatments of adult-onset Still’s disease: An update (in French). Rev Med Interne. 2015; 36: 319–327.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.