Online first
Current issue
Archive
Special Issues
About the Journal
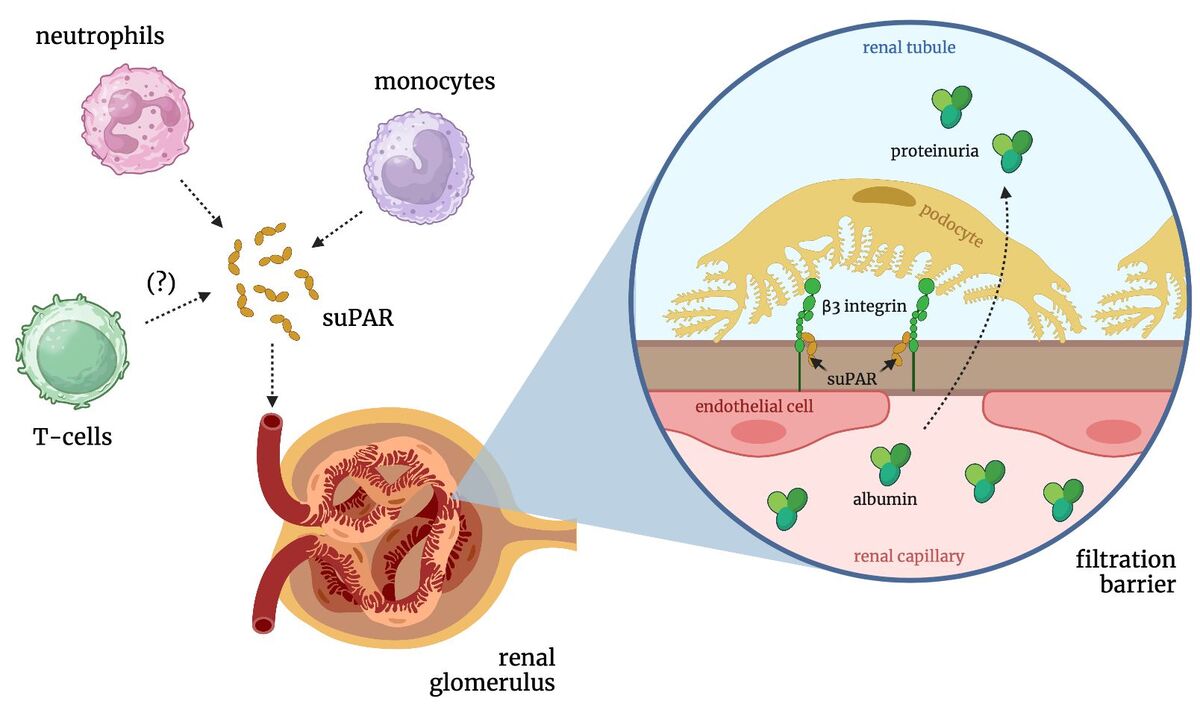
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
A comprehensive review and meta-analysis of suPAR as a predictor of acute kidney injury
1
Medical University, Warsaw, Poland
2
Polish Society of Disaster Medicine, Warsaw, Poland
3
International Academy of Ecology and Medicine, Kyiv, Ukraine
4
Institute of Environmental Protection – National Research Institute, Warsaw, Poland
5
International European University, Kyiv, Ukraine
6
Collegium Medicum, Jan Kochanowski University, Kielce, Poland
7
Baylor College of Medicine, Houston, United States
8
Maria Sklodowska-Curie Medical Academy, Warsaw, Poland
9
Medical University, Lublin, Poland
Ann Agric Environ Med. 2023;30(2):364-368
KEYWORDS
soluble urokinase plasminogen activator receptorsuPARacute kidney injuryacute renal failurebiomarkerpredictionmeta-analysis
TOPICS
Health effects of chemical pollutants in agricultural areas , including occupational and non-occupational effects of agricultural chemicals (pesticides, fertilizers) and effects of industrial disposal (heavy metals, sulphur, etc.) contaminating the atmosphere, soil and waterPrevention of occupational diseases in agriculture, forestry, food industry and wood industryState of the health of rural communities depending on various factors: social factors, accessibility of medical care, etc.
ABSTRACT
Introduction and objective:
The global impact of acute kidney injury (AKI) has not been thoroughly investigated. With the development of new techniques, soluble urokinase plasminogen activator receptor (suPAR) has become increasingly important in the diagnosis of AKI. Therefore, a systematic review and meta-analysis was carried out to evaluate the predictive value of suPAR for AKI.
Material and methods:
The review and meta-analysis investigated the relationship between suPAR levels and acute kidney injury. Pubmed, Scopus, Cochrane Controlled Register of Trials, and Embase were searched for relevant studies from inception to 10 January 2023. Stata (Ver. 16 StataCorp, College Station, TX, USA) was used for all statistical analyses. A random effects model using the Mantel-Haenszel approach was employed, and odds ratios (OR) and standard mean differences (SMD) with 95% confidence intervals (CI) were calculated for binary and continuous outcomes, respectively.
Results:
Nine studies reported suPAR levels among patients with and without AKI. Pooled analysis showed that suPAR levels in patients with and without AKI varied and amounted to 5.23 ± 4.07 vs. 3.23 ±0.67 ng/mL (SMD = 3.19; 95%CI: 2.73 to 3.65; p<0.001). The results from the sensitivity analysis did not alter the direction.
Conclusions:
This results show that increasing suPAR levels are associated with the occurrence of AKI. SuPAR might act as a novel biomarker for CI-AKI in clinical practice.
The global impact of acute kidney injury (AKI) has not been thoroughly investigated. With the development of new techniques, soluble urokinase plasminogen activator receptor (suPAR) has become increasingly important in the diagnosis of AKI. Therefore, a systematic review and meta-analysis was carried out to evaluate the predictive value of suPAR for AKI.
Material and methods:
The review and meta-analysis investigated the relationship between suPAR levels and acute kidney injury. Pubmed, Scopus, Cochrane Controlled Register of Trials, and Embase were searched for relevant studies from inception to 10 January 2023. Stata (Ver. 16 StataCorp, College Station, TX, USA) was used for all statistical analyses. A random effects model using the Mantel-Haenszel approach was employed, and odds ratios (OR) and standard mean differences (SMD) with 95% confidence intervals (CI) were calculated for binary and continuous outcomes, respectively.
Results:
Nine studies reported suPAR levels among patients with and without AKI. Pooled analysis showed that suPAR levels in patients with and without AKI varied and amounted to 5.23 ± 4.07 vs. 3.23 ±0.67 ng/mL (SMD = 3.19; 95%CI: 2.73 to 3.65; p<0.001). The results from the sensitivity analysis did not alter the direction.
Conclusions:
This results show that increasing suPAR levels are associated with the occurrence of AKI. SuPAR might act as a novel biomarker for CI-AKI in clinical practice.
ABBREVIATIONS
AKI – acute kidney injury; ARF – acute renal failure; CI – confidence interval; NGAL – neutrophil gelatinase-associated lipid calin; NOS – Newcastle Ottawa Scale; OR – odds ratio; ROS – reactive oxygen species; SMD – standard mean difference;
suPAR – soluble urokinase plasminogen activator receptor
ACKNOWLEDGEMENTS
The study was financed under the programme of the Ministry of Education and Science in Warsaw in 2019–2023 under the title: ‘Regional Initiative of Excellence’ (Project No. 024/RID/2018/19).
REFERENCES (36)
1.
Rahman M, Shad F, Smith MC. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician. 2012;86:631-639.
2.
Yoon SY, Kim JS, Jeong KH, Kim SK. Acute Kidney Injury: Biomarker-Guided Diagnosis and Management. Medicina (Kaunas) 2022;58(3):340. https://doi.org/10.3390/medici....
3.
Gonsalez SR, Cortês AL, Silva RCD, et al. Acute kidney injury overview: From basic findings to new prevention and therapy strategies. Pharmacol Ther. 2019;200:1-12. https://doi.org/10.1016/j.phar....
4.
Matuszewski M, Ładny J, Rafique Z, et al. Prediction value of soluble urokinase plasminogen activator receptor (suPAR) in COVID-19 patients – a systematic review and meta-analysis. Ann Agric Environ Med. 2023;30:142-147. https://doi.org/10.26444/aaem/....
5.
Sudhini YR, Wei C, Reiser J. suPAR: An Inflammatory Mediator for Kidneys. Kidney Dis (Basel). 2022;8:265-274. https://doi.org/10.1159/000524....
6.
Gussen H, Hohlstein P, Bartneck M, et al. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J Intensive Care. 2019;7:26. https://doi.org/10.1186/s40560....
7.
Hayek SS, Sever S, Ko YA, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015;373:1916-1925. https://doi.org/10.1056/NEJMoa....
8.
Hindy G, Tyrrell DJ, Vasbinder A, et al. Increased soluble urokinase plasminogen activator levels modulate monocyte function to promote atherosclerosis. J Clin Invest. 2022;132:e158788. https://doi.org/10.1172/JCI158....
9.
Nusshag C, Wei C, Hahm E, et al. suPAR links a dysregulated immune response to tissue inflammation and sepsis-induced acute kidney injury. JCI Insight. 2023;8:e165740. https://doi.org/10.1172/jci.in....
10.
Hamie L, Daoud G, Nemer G, et al. SuPAR, an emerging biomarker in kidney and inflammatory diseases. Postgrad Med J. 2018;94:517-524. https://doi.org/10.1136/postgr....
11.
Rasmussen LJH, Petersen JEV, Eugen-Olsen J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front Immunol. 2021;12:780641. https://doi.org/10.3389/fimmu.....
12.
Kim EY, Hassanzadeh Khayyat N, Dryer SE. Mechanisms underlying modulation of podocyte TRPC6 channels by suPAR: Role of NADPH oxidases and Src family tyrosine kinases. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3527-3536. https://doi.org/10.1016/j.bbad....
13.
Piccolella M, Festuccia C, Millimaggi D, et al. suPAR, a soluble form of urokinase plasminogen activator receptor, inhibits human prostate cancer cell growth and invasion. Int J Oncol. 2008;32:185-191. https://doi.org/10.3892/ijo.32....
14.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n7....
15.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. https://doi.org/10.1007/s10654....
16.
Hozo SP, Djulbegovic B, Hozo I.Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. https://doi.org/10.1186/1471-2....
17.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2021;343:d5928. https://doi.org/10.1136/bmj.d5....
18.
Azam TU, Shadid HR, Blakely P, et al. Soluble Urokinase Receptor (SuPAR) in COVID-19-Related AKI. J Am Soc Nephrol. 2020;31:2725-2735. https://doi.org/10.1681/ASN.20....
19.
Hayek SS, Leaf DE, Samman Tahhan A, et al. Soluble Urokinase Receptor and Acute Kidney Injury. N Engl J Med. 2020;382:416-426. https://doi.org/10.1056/NEJMoa....
20.
Mossanen JC, Pracht J, Jansen TU, et al. Soluble Urokinase Plasminogen Activator Receptor and Proenkephalin Serum Levels Predict the Development of Acute Kidney Injury after Cardiac Surgery. Int J Mol Sci. 2017;18:1662. https://doi.org/10.3390/ijms18....
21.
Qin Y, Qiao Y, Wang D, et al. The Predictive Value of Soluble Urokinase-Type Plasminogen Activator Receptor in Contrast-Induced Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Intervention. Int J Gen Med. 2021;14:6497-6504. https://doi.org/10.2147/IJGM.S....
22.
Rasmussen SR, Nielsen RV, Møgelvang R, et al. Prognostic value of suPAR and hsCRP on acute kidney injury after cardiac surgery. BMC Nephrol. 2021;22:120. https://doi.org/10.1186/s12882....
23.
Skalec T, Adamik B, Kobylinska K, et al. Soluble Urokinase-Type Plasminogen Activator Receptor Levels as a Predictor of Kidney Replacement Therapy in Septic Patients with Acute Kidney Injury: An Observational Study. J Clin Med. 2022; 11:1717. https://doi.org/10.3390/jcm110....
24.
Walls AB, Bengaard AK, Iversen E, et al. Utility of suPAR and NGAL for AKI Risk Stratification and Early Optimization of Renal Risk Medications among Older Patients in the Emergency Department. Pharmaceuticals (Basel). 2021;14:843. https://doi.org/10.3390/ph1409....
25.
Zhang Y, Zhao H, Su Q, et al. Novel Plasma Biomarker-Based Model for Predicting Acute Kidney Injury After Cardiac Surgery: A Case Control Study. Front Med (Lausanne). 2022;8:799516. https://doi.org/10.3389/fmed.2....
26.
Aronen A, Aittoniemi J, Huttunen R, et al. Preoperative plasma soluble urokinase plasminogen activator receptor as a prognostic marker in rectal cancer patients. An EORTC-Receptor and Biomarker Group collaboration. Int J Biol Markers. 2005;20:93-102. https://doi.org/10.5301/JBM.20....
27.
Uzawa A, Kojima Y, Ozawa Y, et al. Serum level of soluble urokinase plasminogen activator receptor (suPAR) as a disease severity marker of myasthenia gravis: a pilot study. Clin Exp Immunol. 2020;202:321-324. https://doi.org/10.1111/cei.13....
28.
Şirinoğlu M, Soysal A, Karaaslan A, et al. The diagnostic value of soluble urokinase plasminogen activator receptor (suPAR) compared to C-reactive protein (CRP) and procalcitonin (PCT) in children with systemic inflammatory response syndrome (SIRS). J Infect Chemother. 2017;23:17-22. https://doi.org/10.1016/j.jiac....
29.
Hoenigl M, Raggam RB, Wagner J, et al. Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammatory response syndrome. Clin Biochem. 2013;46:225-229. https://doi.org/10.1016/j.clin....
30.
Su L, Zhang J, Peng Z. The role of kidney injury biomarkers in COVID-19. Ren Fail. 2022;44:1280-1288. https://doi.org/10.1080/088602....
31.
Hall A, Crichton S, Varrier M, et al. suPAR as a marker of infection in acute kidney injury - a prospective observational study BMC Nephrol. 2018;19:191. https://doi.org/10.1186/s12882....
32.
Huang Y, Huang S, Zhuo X, et al. Predictive value of suPAR in AKI: a systematic review and meta-analysis. Clin Exp Nephrol. 2023;27:1-11. https://doi.org/10.1007/s10157....
33.
Tavenier J, Haupt TH, Andersen AL, et al. A high-protein diet during hospitalization is associated with an accelerated decrease in soluble urokinase plasminogen activator receptor levels in acutely ill elderly medical patients with SIRS. Nutr Res. 2017;41:56-64. https://doi.org/10.1016/j.nutr....
34.
Iversen E, Kallemose T, Hornum M, et al. Soluble urokinase plasminogen activator receptor and decline in kidney function among patients without kidney disease. Clin Kidney J. 2022;15:1534-1541. https://doi.org/10.1093/ckj/sf....
35.
Griffin BR, Gist KM, Faubel S.Current status of novel biomarkers for the diagnosis of acute kidney injury: a historical perspective. J Intensive Care Med. 2020;35:415–424. https://doi.org/10.1177/088506....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.