Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
CASE REPORT
Candida glabrata as an aetiological factor of the fulminant course of panophthalmitis
1
Department of Maxillofacial Surgery, Heliodor Święcicki Clinical Hospital, University of Medical Sciences, Poznań, Poland
2
Central Microbiology Laboratory, H. Swiecicki Clinical Hospital, University of Medical Sciences, Poznań, Poland
3
Department of Genetics and Pharmaceutical Microbiology, University of Medical Sciences, Poznań, Poland
4
Department of Dermatology and Venereology, University of Medical Sciences, Poznań, Poland
Corresponding author
Joanna Bilska-Stokłosa
Department of Maxillofacial Surgery, Heliodor Święcicki Clinical Hospital. Poznan University of Medical Sciences, Poland, ul. Przybyszewskiego 49, 60-355, Poznań
Department of Maxillofacial Surgery, Heliodor Święcicki Clinical Hospital. Poznan University of Medical Sciences, Poland, ul. Przybyszewskiego 49, 60-355, Poznań
Ann Agric Environ Med. 2020;27(4):540-543
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The role of fungi in infections in immunocompromised patients is a growing problem in both diagnosis and treatment. Candida species are the most common cause of fungal, endogenous endophthalmitis and infections of the cornea.
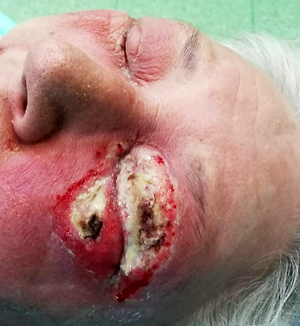
Case study:
A patient was admitted to hospital due to acute inflammation of the tissue of the left orbit, 1.5 years after the corneal penetrating transplantation of the left eye with intracapsular extraction of lens and simultaneous anterior vitrectomy. The microbiological system identified: Streptococcus pyogenes, Staphylococcus aureus, and Candida glabrata in the patient.
Conclusions:
The factors conducive to fungal infections are: patient’s old age, immune disorders and diabetes, as well as the presence of a necrotic tissue or a foreign body. All these parameters were met in this case. Only antibiotic therapy and long-term antifungal therapy, together with surgical debridement of the site of the ongoing infection produces clinical effects in such severe cases.
The role of fungi in infections in immunocompromised patients is a growing problem in both diagnosis and treatment. Candida species are the most common cause of fungal, endogenous endophthalmitis and infections of the cornea.
Case study:
A patient was admitted to hospital due to acute inflammation of the tissue of the left orbit, 1.5 years after the corneal penetrating transplantation of the left eye with intracapsular extraction of lens and simultaneous anterior vitrectomy. The microbiological system identified: Streptococcus pyogenes, Staphylococcus aureus, and Candida glabrata in the patient.
Conclusions:
The factors conducive to fungal infections are: patient’s old age, immune disorders and diabetes, as well as the presence of a necrotic tissue or a foreign body. All these parameters were met in this case. Only antibiotic therapy and long-term antifungal therapy, together with surgical debridement of the site of the ongoing infection produces clinical effects in such severe cases.
Bilska-Stokłosa J, Hampelska K, Osmola K, Czajka J, Bogdanowicz-Gapińska D, Tomczak H. Candida glabrata as an aetiological factor of the
fulminant course of panophthalmitis. Ann Agric Environ Med. 2020; 27(4): 540–543. doi: 10.26444/aaem/122475
REFERENCES (23)
1.
Benamu E, Hogan C.A, Gomez C.A. Endemic Fungi in Transplant and Immunocompromised Hosts: Epidemiology, Diagnosis, Treatment, and Prevention. Cur Treat Options Infect Dis. 2020; 1–24.
2.
Kalkanci A, Sengul O. Ocular fungal infections. Current Eye Res. 2011; 179–189.
3.
Seidelman J. 1688. Invasive Ocular Candidiasis: Who Is Really at Risk? Open Forum Infectious Diseases. 2019.
4.
Durand ML. Bacterial and fungal endophthalmitis. Clin Microbiol Rev. 2017; 597–613.
5.
Vos FI. Eye for an eye: near-fatal outcome of fungal infection in a young, diabetic girl. Case Reports. 2018.
6.
Berkow EL, Lockhart SR. Fluconazole resistance in Candida species: a current perspective Infect Drug Resist. 2017; 10: 237–245. doi: 10.2147/IDR.S118892.
7.
Watkins R R. Isavuconazole vs. Caspofungin for Candidemia. Infectious Disease Alert. 2019; 38: 11.
8.
Kim J. A Randomized, Double-Blind Study to Evaluate the Safety, Tolerability, and Efficacy of Caspofungin vs. Amphotericin B Deoxycholate in the Treatment of Invasive Candidiasis in Neonates and Infants≤ 3 Months of Age. Open Forum Infectious Diseases. Oxford University Press. 2018; 565.
9.
Mukherjee PK. Candida biofilm: a well-designed protected environment. Medical Mycology. 2005; 43(3): 191–208.
10.
Silva S. Candida species biofilms’antifungal resistance. J Fungi. 2017; 3(1): 8.
11.
Taff H. T. Mechanisms of Candida biofilm drug resistance. Future Microbiology. 2013; 8(10): 1325–1337.
12.
Tsui C, Kong EF, Jabra-Rizk MA. Pathogenesis of Candida albicans biofilm. FEMS Pathogens and Disease. 2016; 74(4).
13.
Cunha BA. Sepsis and its mimics in the critical care unit. Infectious Diseases in Critical Care Medicine. 2nd ed. (NY) Publishers, 2016.
14.
Reiss-Mandel A, Regev-Yochay G. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: myth or reality? Human vaccines & immunotherapeutics. 2016; 12(2): 351–357.
15.
Laux C, Peschel A, Krismer B. Staphylococcus aureus colonization of the human nose and interaction with other microbiome members. Gram-Positive Pathogens. 2019; 723–730.
16.
Otto M. Staphylococcus aureus toxins. Current opinion in microbiology. 2014; 17: 32–37.
18.
Dudiuk C, Berrio I, Leonardelli F, Morales-Lopez S, Theill L, Macedo D, Garcia-Effron G. Antifungal activity and killing kinetics of anidulafungin, caspofungin and amphotericin B against Candida auris. Journal of Antimicrobial Chemotherapy. 2019; 74(8): 2295–2302.
19.
Reijtman V. Evaluation of a rapid diagnostic test for the detection of Streptococcus pyogenes in invasive infections. Revista Argentina de Microbiología, 2020.
20.
Devaere M, Boukthir S, Moullec S. Complete Genome Sequences of Two Strains of Streptococcus pyogenes Belonging to an Emergent Clade of the Genotype emm89 in Brittany, France. Microbiol Resour Announc. 2020; 9(11): e00129–20. https://doi.org/10.1128/MRA.00....
22.
Priya A, Shunmugiah KP. Bacteriology of Ophthalmic Infections. Pocket Guide to Bacterial Infections. CRC Press. 2019; 319–363.
23.
Pham CD. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrobial agents and chemotherapy. 2014; 58(8): 4690–4696.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.