Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
BRIEF COMMUNICATION
Molecular detection of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) in eastern Poland
1
Faculty of Veterinary Medicine, University of Life Sciences, Lublin, Poland
2
University of Cordoba, Spain
Corresponding author
Lukasz Adaszek
University of Life Sciences, Faculty of Veterinary Medicine, Głęboka 30, 20-612, Lublin, Poland
University of Life Sciences, Faculty of Veterinary Medicine, Głęboka 30, 20-612, Lublin, Poland
Ann Agric Environ Med. 2020;27(4):702-705
KEYWORDS
TOPICS
ABSTRACT
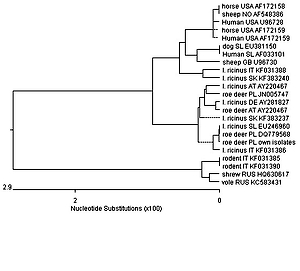
Wild ungulates may serve as reservoirs for particular tick-borne pathogens (TBP) of importance for public and animal health. The aim of the study was to investigate, for the first time, the occurrence of Anaplasma phagocytophilum (AP) in a large population of roe deer in eastern Poland. Spleen samples from 424 roe deer (Capreolus capreolus) were collected during the 2018–2019 hunting season. Genetic screening for AP was carried out by PCR amplification of a fragment of the 16S rRNA and groEL genes. Twenty-six of the 424 spleen samples (6.13%) tested positive both for 16S rRNA and groEL. Phylogenetic analysis confirmed the existence of a specific roe deer ecotype of AP in eastern Poland. Despite the low prevalence of AP in roe deer populations in the study area, these animals may act as anaplasmosis reservoirs for ticks. Based on the zoonotic potential of granulocytic anaplasmosis, it seems advisable to introduce continuous monitoring of this infection among domestic, farm and wild animals.
CONFLICT OF INTEREST
The authors declare that they have no competing interests. The authors certify that they have no affiliation with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript
Teodorowski O, Radzki R, Kalinowski M, Winiarczyk S, Garcia Bocanegra I, Winiarczyk D, Adaszek L. Molecular detection of Anaplasma
phagocytophilum in roe deer (Capreolus capreolus) in eastern Poland. Ann Agric Environ Med. 2020; 27(4): 702–705. doi: 10.26444/aaem/124902
REFERENCES (36)
1.
Vayssier-Taussat M, Kazimirova M, Hubalek Z, Hornok S, Farkas R, Cosson JF, et al. Emerging horizons for tick-borne pathogens: from the ‘one pathogen-one disease’ vision to the pathobiome paradigm. Future Microbiol. 2015; 10(12): 2033–2043. https://doi.org/10.2217/fmb.15....
2.
Munderloh U. Comparative studies in tick-borne diseases in animals and humans. Vet Sci. 2017; 4(2): E32. https://doi.org/10.3390/vetsci....
3.
Asman M, Witecka J, Solarz K, Zwonik A, Szilman P. Occurrence of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in Ixodes ricinus ticks collected from selected areas of Opolskie Province in south-west Poland. Ann Agric Environ Med. 2019; 26(4): 544–547. https://doi.org/10.26444/aaem/....
4.
Mysterud A, Stigum VM, Seland IV, Herland A, Easterday WR, Jore S, et al. Tick abundance, pathogen prevalence, and disease incidence in two contrasting regions at the northern distribution range of Europe. Parasit Vectors. 2018; 11(1): 309. https://doi.org/10.1186/s13071....
5.
Battilani M, De Arcangeli S, Balboni A, Dondi F. Genetic diversity and molecular epidemiology of Anaplasma. Infect Genet Evol. 2017; 49: 195–211. https://doi.org/10.1016/j.meeg....
6.
Kocan KM, de la Fuente J, Coburn LA. Insights into the development of Ixodes scapularis: a resource for research on a medically important tick species. Parasit Vectors. 2015; 8: 592. https://doi.org/10.1186/s13071....
7.
Dzięgiel B, Adaszek Ł, Winiarczyk S. Wild animals as reservoirs of Anaplasma phagocytophilum for humans. Przegl Epidemiol. 2016; 70(3): 428–443.
8.
Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 2012; 28(10): 437–446. https://doi.org/10.1016/j.pt.2....
9.
Bakken JS, Krueth JK, Lund T, Malkovitch D, Asanovich K, Dumler JS. Exposure to deer blood may be a cause of human granulocytic ehrlichiosis. Clin Infect Dis. 1996; 23(1): 198. https://doi.org/ 10.1093/clinids/23.1.198.
10.
Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Heylen D, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014; 15; 7: 365. https://doi.org/10.1186/1756-3....
11.
Rymaszewska A. Divergence within the marker region of the groESL operon in Anaplasma phagocytophilum. Eur J Clin Microbiol Infect Dis. 2008; 27(11): 1025–1036. https://doi.org/ 10.1007/s10096-008-0539-x.
12.
Hapunik J, Víchová B, Karbowiak G, Wita I, Bogdaszewski M, Pet’ko B. Wild and farm breeding cervids infections with Anaplasma phagocytophilum. Ann Agric Environ Med. 2011; 18(1): 73–77.
13.
Adaszek Ł, Klimiuk P, Skrzypczak M, Górna M, Ziętek J, Winiarczyk S. The identification of Anaplasma spp. isolated from fallow deer (Dama dama) on a free-range farm in eastern Poland. Pol J Vet Sci. 2012; 15(2): 393–394. https://doi.org/10.2478/v10181....
14.
Michalik J, Stańczak J, Cieniuch S, Racewicz M, Sikora B, Dabert M. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg Infect Dis. 2012; 18(6): 998–1001. https://doi.org/10.3201/eid180....
15.
Dzięgiel B, Adaszek Ł, Krzysiak M, Skrzypczak M, Adaszek M, Furmaga B, et al. The occurrence of Anaplasma phagocytophilum in wild bison from Białowieza Primeval Forest in Eastern Poland. Berl Münch Tierärztl Wochenschr. 2015; 128(7–8): 310–314.
16.
Szewczyk T, Werszko J, Myczka AW, Laskowski Z, Karbowiak G. Molecular detection of Anaplasma phagocytophilum in wild carnivores in north-eastern Poland. Parasit Vectors. 2019; 12(1): 465. https://doi.org/10.1186/s13071....
17.
Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, et al. Nested PCR assay for detection of granulocytic ehrlichiae. J Clin Microbiol. 1998; 36(4): 1090–1095.
18.
Alberti A, Zobba R, Chessa B, Addis MF, Sparagano O, Pinna Parpaglia ML, et al. Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl Environ Microbiol. 2005; 71(10): 6418–6422. https://doi.org/10.1128/AEM.71....
19.
Adaszek Ł, Winiarczyk S, Łukaszewska J. A first case of ehrlichiosis in a horse in Poland. Dtsch Tierarztl Wochenschr. 2009; 116(9): 330–334. https://doi.org/10.2376/0341-6....
20.
Hartwig V, von Loewenich FD, Schulze C, Straubinger RK, Daugschies A, Dyachenko V. Detection of Anaplasma phagocytophilum in red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) from Brandenburg, Germany. Ticks Tick Borne Dis. 2014; 5(3): 277–280. https://doi.org/10.1016/j.ttbd....
21.
Michel AO, Mathis A, Ryser-Degiorgis MP. Babesia spp. in European wild ruminant species: parasite diversity and risk factors for infection. Vet Res. 2014; 45: 65. https://doi.org/10.1186/1297-9....
22.
Messner M, Kayikci FN, Shahi-Barogh B, Harl J, Messner C, Fuehrer HP. Screening of wild ruminants from the Kaunertal and other alpine regions of Tyrol (Austria) for vector-borne pathogens. Parasitol Res. 2019; 118(9): 2735–2740. https://doi.org/10.1007/s00436....
23.
Blaňarová L, Stanko M, Carpi G, Miklisová D, Víchová B, Mošanský L, et al. Distinct Anaplasma phagocytophilum genotypes associated with Ixodes trianguliceps ticks and rodents in Central Europe. Ticks Tick Borne Dis. 2014; 5(6): 928–938. https://doi.org/10.1016/j.ttbd....
24.
Kauffmann M, Rehbein S, Hamel D, Lutz W, Heddergott M, Pfister K, et al. Anaplasma phagocytophilum and Babesia spp. in roe deer (Capreolus capreolus), fallow deer (Dama dama) and mouflon (Ovis musimon) in Germany. Mol Cell Probes. 2017; 31: 46–54. https://doi.org/10.1016/j.mcp.....
25.
Bown KJ, Begon M, Bennett M, Woldehiwet Z, Ogden NH. Seasonal dynamics of Anaplasma phagocytophila in a rodent-tick (Ixodes trianguliceps) system, United Kingdom. Emerg Infect Dis. 2003; 9(1): 63–70. https://doi.org/10.3201/eid090....
26.
Adamska M, Skotarczak B. Wild game as a reservoir of Anaplasma phagocytophilum in north-western Poland. Wiad Parazytol 2007; 53(2): 103–107.
27.
Jouglin M, Blanc B, de la Cotte N, Bastian S, Ortiz K, Malandrin L. First detection and molecular identification of the zoonotic Anaplasma capra in deer in France. PLoS One. 2019; 14(7): e0219184. https://doi.org/10.1371/journa....
28.
Di Domenico M, Pascucci I, Curini V, Cocco A, Dall’Acqua F, Pompilii C, et al. Detection of Anaplasma phagocytophilum genotypes that are potentially virulent for human in wild ruminants and Ixodes ricinus in Central Italy. Ticks Tick Borne Dis. 2016; 7(5): 782–787. https://doi.org/10.1016/j.ttbd....
29.
Welc-Falęciak R, Werszko J, Cydzik K, Bajer A, Michalik J, Behnke JM. Co-infection and genetic diversity of tick-borne pathogens in roe deer from Poland. Vector Borne Zoonotic Dis. 2013; 13(5): 277–288. https://doi.org/10.1089/vbz.20....
30.
Overzier E, Pfister K, Herb I, Mahling M, Böck G Jr, Silaghi C. Detection of tick-borne pathogens in roe deer (Capreolus capreolus), questing ticks (Ixodes ricinus) and ticks infesting roe deer in southern Germany. Ticks Tick Borne Dis. 2013; 4(4): 320–328. https://doi.org/10.1016/j.ttbd....
31.
Dzięgiel B, Adaszek Ł, Carbonero A, Łyp P, Winiarczyk M, Dębiak P, et al. Detection of canine vector-borne diseases in eastern Poland by ELISA and PCR. Parasitol Res. 2016; 115(3): 1039–1044. https://doi.org/ 10.1007/s00436-015-4832-1.
32.
Kazimírová M, Hamšíková Z, Špitalská E, Minichová L, Mahríková L, Caban R, et al. Diverse tick-borne microorganisms identified in free-living ungulates un Slovakia. Parasit Vectors. 2018; 11(1): 495.https://doi.org/10.1186/s13071....
33.
de la Fuente J, Gortazar C. Wild boars as hosts of human-pathogenic Anaplasma phagocytophilum variants. Emerg Infect Dis. 2012; 18(12): 2094–2095. https://doi.org/10.3201/eid181....
34.
Galindo RC, Ayllon N, Smrdel KS, Boadella M, Beltran-Beck B, Mazariegos M, et al. Gene expression profile suggests that pigs (Sus scrofa) are susceptible to Anaplasma phagocytophilum but control infection. Parasit Vectors. 2012; 5: 181. https://doi.org/10.1186/1756-3....
35.
Atif FA. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol Res. 2015; 114(11): 3941–3957. https://doi.org/10.1007/s00436....
36.
Matei IA, Estrada-Peña A, Cutler SJ, Vayssier-Taussat M, Varela-Castro L, Potkonjak A, et al. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasit Vectors. 2019; 12(1): 599. https://doi.org/10.1186/s13071....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.